NeuClone discloses its sixth biosimilar candidate of Pertuzumab
December 18, 2018 | Tuesday | News
NeuClone's Perjeta (pertuzumab) biosimilar is partnered with Serum Institute of India (Serum). Under the partnership, NeuClone and Serum are developing ten biosimilars for global markets.
NeuClone Pharmaceuticals Ltd. (NeuClone), a clinical-stage biopharmaceutical company exclusively focused on developing high-quality biosimilar products has disclosed the sixth biosimilar being developed in its pipeline of monoclonal antibody (mAb) products. The product is a biosimilar candidate of Perjeta (pertuzumab) currently in preclinical development.
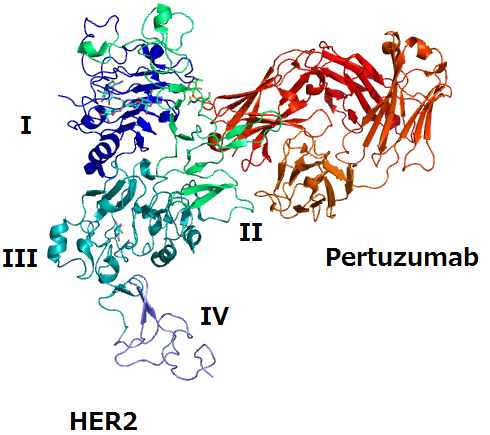
Perjeta (pertuzumab) is FDA and EMA approved as a treatment for patients with HER2-positive early or metastatic breast cancer and is authorised for use only in combination with trastuzumab. In addition to the pertuzumab biosimilar, NeuClone is also developing a biosimilar of Herceptin (trastuzumab) currently in clinical development.
NeuClone's Perjeta (pertuzumab) biosimilar is partnered with Serum Institute of India (Serum). Under the partnership, NeuClone and Serum are developing ten biosimilars for global markets.
"We believe biosimilar combination therapies referencing Perjeta and Herceptin represent an exciting development in the future of biosimilars," stated Dr. Noelle Sunstrom, CEO of NeuClone. "Current combination treatments of mAbs, while often clinically superior to monotherapies, are extremely expensive when available. We are determined to dramatically expand the number of patients able to receive these life-changing combination therapies by offering lower-priced biosimilars of both mAbs."
"Biosimilars are fast becoming a very important part of our pipeline," says Adar C. Poonawalla, CEO of Serum Institute of India. "NeuClone's biosimilar technology coupled with our low-cost, large-scale business model, will enable us to supply these critical antibody therapies to those previously unable to receive treatment."
Pertuzumab is an IgG1 humanized mAb that targets human epidermal growth factor receptor 2 (HER2). Approximately 20% of women diagnosed with breast cancer test positively for HER2. According to La Merie, Perjeta recorded global sales of $2.3 billion in 2017.
Dr. Noelle Sunstrom and other NeuClone representatives will be attending the 37th Annual J.P. Morgan Healthcare Conference in San Francisco from 7-10th January 2019. As part of NeuClone's strategy for biosimilar products the company is seeking commercialisation partners.