Merck lifts up its BioContinuum™ Platform strategy
April 03, 2019 | Wednesday | News
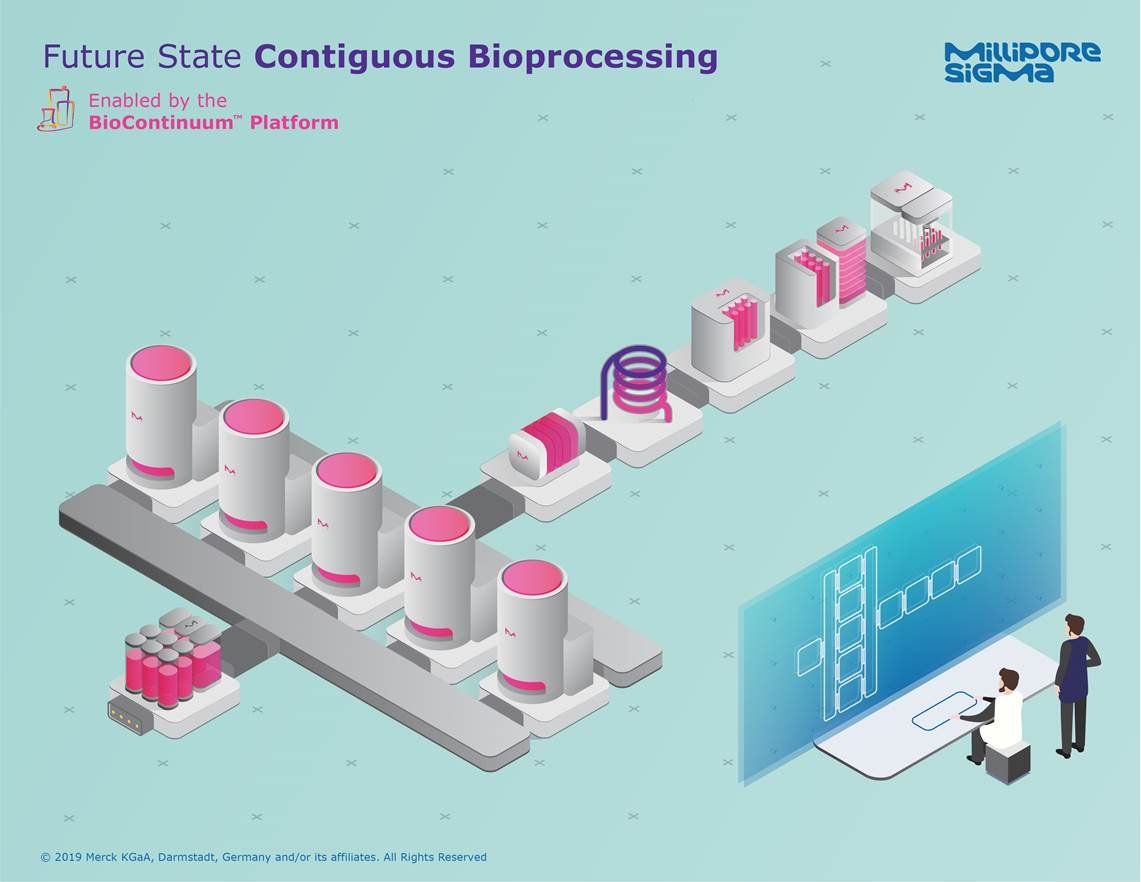
“ContiGuous” processing will deliver seamless physical and digital integration of BioContinuum™ Platform building blocks
Merck discloses it’s BioContinuum™ Buffer Delivery Platform, a new building block in the BioContinuum™ Platform for next-generation bioprocessing, at INTERPHEX in New York City. The BioContinuum™ Buffer Delivery Platform is part of Merck’s BioContinuum™ Platform to deliver “contiGuous” bioprocessing.
The BioContinuum™ Buffer Delivery Platform is an integrated solution for more efficient buffer delivery and is tailored to provide the highest level of accuracy and precision in buffer preparation and management. Its launch is timely, as industry adoption of liquid buffers is expected to expand from seven to 56 percent, according to Merck’s internal market research.
Merck has obtained certain exclusive rights to sell the buffer dilution system developed by LEWA-Nikkiso America Inc (now a part of YMC Co., Ltd.) for customers involved in the development or production of diagnostics and biopharmaceuticals. This buffer dilution system offers the highest accuracy available for dilution of highly concentrated buffer, and Merck is the only supplier providing such an integrated offering. Merck’s BioContinuum™ Buffer Delivery Platform supplies process buffers at a fraction of the resources and facility space, resulting in a more streamlined buffer suite and a more efficient manufacturing process.
The configurable platform is comprised of four components: a selection of distinct buffer concentrates, reliably delivered in Mobius® Select single-use assemblies and an automated, highly accurate and precise buffer dilution system with tailored system services.
ContiGuous manufacturing goes beyond just connecting the individual unit operations — it will include the orchestration and management of all the processing steps (materials, production, testing and analytics) with an industry-leading streamlined and optimized approach.
Merck also introduced Emprove® Evolve, a new product line under the Emprove® Program. Emprove® Evolve provides a new area of regulatory support in risk assessment for critical raw materials, helping drug manufacturers meet developing regulatory requirements. The program contains a high level of product characterization and documentation, providing more control of the manufacturing process and allowing manufacturers to select the appropriate quality of raw materials for drug development.