AMPEL BioSolutions brings new hope for patients suffering from gout
January 03, 2019 | Thursday | News
While many treatments lower urate levels, there previously has been none proven to rapidly eliminate the body burden of urate that causes the signs and symptoms of gout
AMPEL Bio Solutions, a leading technology research firm based in Charlottesville, VA today announced the publication of new data in Arthritis Research and Therapy, co-authored by AMPEL Co-Founder Dr. Peter Lipsky, which demonstrates the effectiveness of Pegloticase (Horizon Pharma) in the resolution of urate depositions or tophi in people suffering from Gout.
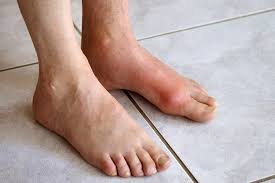
Gout is an ancient disease and the most common form of inflammatory arthritis in the US, with more than 8 million affected people. Gout is caused by the deposition of urate crystals into tissues. It is a genetically-based metabolic and inflammatory condition that can be worsened by diet, kidney disease and certain medications. Gout can cause inflammatory arthritis and is associated with many other diseases, including cardiovascular and kidney disease. The cardinal feature of Gout is the deposition of urate crystals into aggregates known as tophi, which can cause painful joint swelling.
Numerous treatments are available to decrease urate levels, but most are very slow acting and take years to resolve urate build up and cause visible clinical benefits. In contrast, Pegloticase has been proven to rapidly degrade urate levels, giving hope for a new thorough and proactive treatment for Gout patients.
It is an innovative treatment that acts as a replacement therapy for the uricase enzyme that humans lost through evolution. Acting as the uricase enzyme, Pegloticase rapidly and persistently decreases urate levels inside the body and simultaneously resolves visible tophi and joint swelling that result from those urate deposits.
Dr. Brian Mandell, a steering committee member of Gout Study Group, said, "Other than for acute attacks, it is no surprise that physicians have a no rush approach to the treatment of Gout. This has been reinforced over the years by the absence of any effective therapy to rapidly dissolve these tophi. Yet some patients are profoundly incapacitated by the presence of tophi not associated with flare-ups, that cannot be seen by the naked eye, and that prevent the use of their hands, or drain through chronically infected sinus tracts. These patients, and those suffering from flare-ups can benefit enormously from rapid tophi resolution. In the current reanalysis of data from the initial trials of Pegloticase, there is proof of concept that by maintaining an extremely low serum urate level over the course of only months, tophi can be very rapidly and in many patients completely dissolved.
By using its unique data mining capability, AMPEL was able to show that the administration of Pegloticase caused a complete resolution of all visible tophi in a mean of 9 months. This is dramatically faster than with other available urate-lowering drugs that work by other mechanisms and may take years to resolve tophi. Since tophi are associated with local bone damage, loss of function when affecting the hand and also a decreased quality of life, rapid resolution of tophi provides new hope for the many people suffering from gout."
Dr. Peter Lipsky, Co-founder of AMPEL, said, "the results published today demonstrate and validate AMPEL's novel approach to data mining as a tool in understanding the effectiveness of a drug. In this case AMPEL's deep mining of data from the randomized controlled trials of Pegloticase, a U.S. approved treatment for chronic Gout, demonstrated that this treatment is proven to lower urate levels dramatically faster than the standard of care, given patients relief from both visible and lurking urate deposits."