How can Dispensers combat counterfeit medicines?
April 23, 2021 | Friday | Views
Malpractitioners continue to replicate and market counterfeits
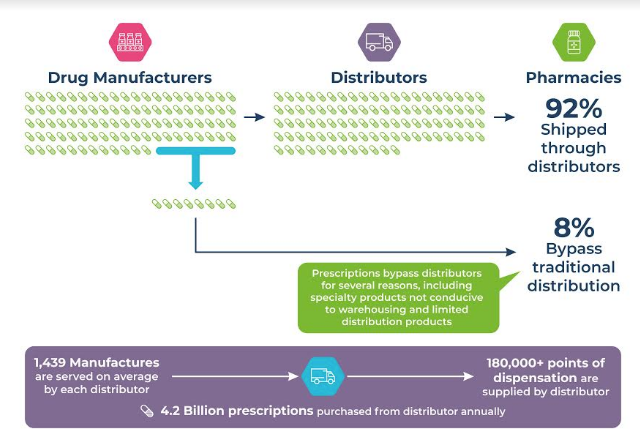
image caption- Role of distributors in the supply chain
Most counterfeit products target critical medication such as immune-suppressants, HIV medication, heart medication, and injectable (biologics) for which the unit price is high.
We are also seeing counterfeit versions of essentials such as strips for monitoring sugar levels, vaccines, and antiallergens. Other cases of counterfeit products in the market include the resale of expired legitimate brands.
In such cases, the expiry date is tampered with and marked to indicate the product is safe for consumption. Using online marketing, gray pharmaceutical marketing counterfeits often reach the end consumer through legitimate transit networks and retailers.
Despite the efforts taken by manufacturers to differentiate legitimate products from counterfeits, such as using unique packing methods, manufacturer specific holograms, and unique labeling, malpractitioners continue to replicate and market counterfeits.
Pharma supply chain
The pharmaceutical supply chain is complex and begins with the product originating at the manufacturing site, before being transferred to distributors (wholesalers and retailers) and, finally, the end dispensers.
A study by the Health Care Distribution Alliance (HDA) in 2019, emphasized the role of distributers in the pharma supply chain. The study deduced that the supply chain distribution cycle from the manufacturer through distributers, to the end dispenser accounts for some 92 percent of the flow of medicine to the end customer. The remaining 8 percent is directly routed from the Manufacturers to the end dispensers and, from there, to the end customer. Establishing, the need for traceability of the transacted product(s) through to the end dispenser(s) is essential.
Current controls the dispensers maintain
A dispenser may be a hospital, a pharmacy, or an individual(s) who hold a license(s) to commercially dispense prescription drugs to a patient.
The risk of counterfeit products penetrating the end dispenser may occur due to incomplete traceability. This means that the exact information of which product(s) handed over as part of sale cannot be entirely traced back to the manufacturer. With the number of intermediaries within the supply chain from the manufacturer to the dispenser, it is imperative to only engage in trade with authorized trading partners.
The first requirement set for dispensers was in 2015, this mandated that dispensers only trade with Authorized trading partners. To do so they must validate the trading partner as authorized prior to engaging in a transaction for purchase and/or for sale .
In addition, accepting ownership of a product from an upstream partner can only happen if the T3 information: Transaction Information (TI), Transaction History (TH), and Transaction Statement (TS), as outlined by the Drug Supply Chain Security Act (DSCSA), are available.
The T3 identifies the chain of ownership for the product which can be tracked back to the manufacturer, this ensures traceability of ownership for the incoming goods. The supply of T3 information is enforced for the dispensers as well, in case of the sale of product from one dispenser to another, which enforces a change in ownership. This process is currently in effect for the upstream and downstream trade of product(s) with all dispenser transactions.
2020 saw further requirements for dispensers come into force, ensuring that they only engage in the trade of serialized products. Serialization of product enables product traceability.
This means that, ‘Dispenser transactions’ can only be made if the product is serialized, the only exception is in the trading of a grandfathered product with verifications in place that ensure the T3 information and the trading partners are authorized.
Dispensers should have the capability to scan and verify serialized saleable units by parsing the 2D data matrix barcodes affixed by the manufacturers. Additional advantages in keeping track of serialized inventory enables the dispenser to identify serials sourced out in transactions.
A delay has been enforced on the requirement relating to the verification of package identifiers in the case of a suspect product investigation. This is due to the readiness of dispensers, not having the necessary capabilities in place to carry out the verification. Owing to the delay, the 2020 requirements for a dispenser are expected to be achieved by the final DSCSA deadline of 2023.
Conclusion
With the serialization efforts already in place at the start of the supply chain (manufacturers and distributors), there are technologies available that help maintain the serialized information while, at the same time, offering flexible solutions for organizations to include business-specific workflows.
What we see are a number of options now available to enable the dispensing sector to, not only, manage serialized inventory, based on extensibility of use, also to verify authorized trading partners electronically as part of a verification workflow.
With a lead-time of 2023, along with existing technologies for maintaining serialized information, there is a good window for dispensers to analyze and adopt existing technologies based on their business workflows to be ready for a fully interoperable supply chain.
Krishnan S, General Manager, Consulting, Navitas Life Sciences; Santosh Narayanan BT, Senior Business Analyst, Consulting, Navitas Life Sciences