Medtronic helps reduce infections associated with cardiac implants
February 25, 2021 | Thursday | News
Launches TYRX™ Absorbable Antibacterial Envelope (TYRX Envelope) - an absorbable, single-use, antibacterial envelope designed to stabilize a cardiac implantable electronic device (CIED) or implanted neurostimulator
Source: businesswireindia.com
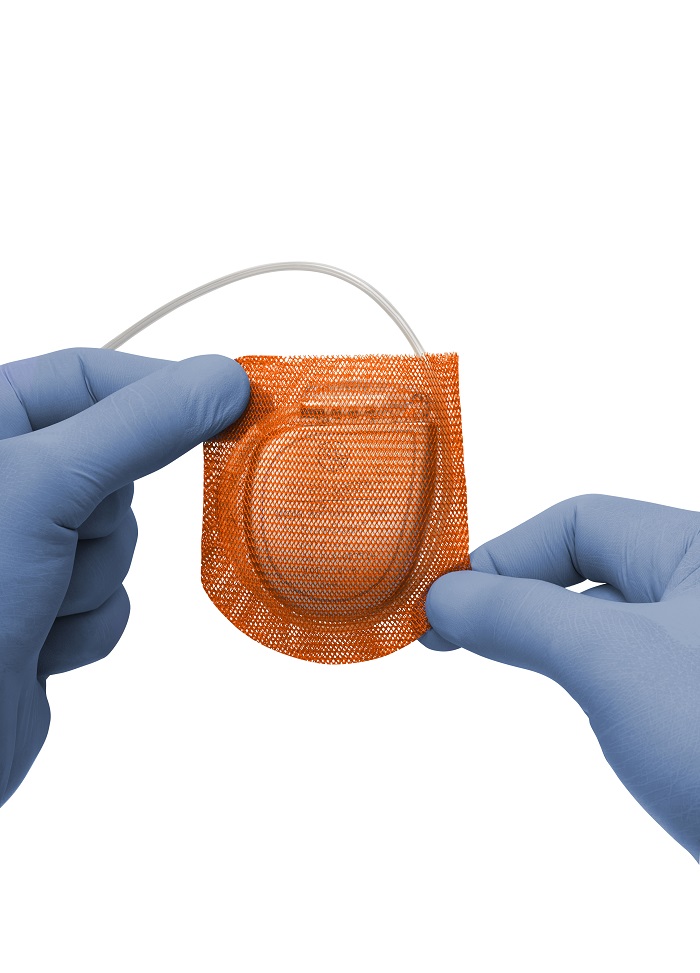
India Medtronic Private Limited, a wholly owned subsidiary of Medtronic plc has announced the launch of the TYRX™ Absorbable Antibacterial Envelope (TYRX Envelope) - an absorbable, single-use, antibacterial envelope designed to stabilise a cardiac implantable electronic device (CIED) or implanted neurostimulator while releasing antimicrobial agents over a minimum of seven days.
Constructed from a multifilament, knitted absorbable mesh, the TYRX Envelope holds the CIED device and is fully absorbed by the body approximately nine weeks after implantation. It can be used with any implantable defibrillator, pacemaker, or neurostimulator.
“We strive to offer products and services of the highest quality that deliver clinical and economic value to patients and physicians. TYRX is one such value-based offering aimed at lowering infection risk and reducing readmission rates with the long-term goal of creating better outcomes,” said Abhishek Bhargava, Director, Cardiac Rhythm Management, Cardiac Ablation & Diagnostics, Medtronic India.
Results from the landmark Worldwide Randomised Antibiotic Envelope Infection Prevention Trial (WRAP-IT) demonstrated the TYRX Envelope reduced the risk of major infection by 40 per cent in patients with CIEDs, and reduced pocket infections by 61 per cent, when used as an adjunctive therapy in addition to standard-of-care infection prevention strategies for patients at higher risk of infection. The study enrolled approximately 7,000 patients, from 181 centres in 25 countries in Asia, Europe, North America, and South America.
The study population included patients receiving an initial cardiac resynchronisation therapy defibrillator (CRT-D); and patients receiving a replacement, system revision or generator upgrade of an existing pacemaker, cardiac resynchronisation therapy-pacemaker (CRT-P), implantable cardioverter defibrillator (ICD) or CRT-D.
The TYRX Envelope was cleared by the FDA in 2013 and received CE Mark in 2014.