Meril launches first indigenously developed TAVR therapy
December 10, 2018 | Monday | News
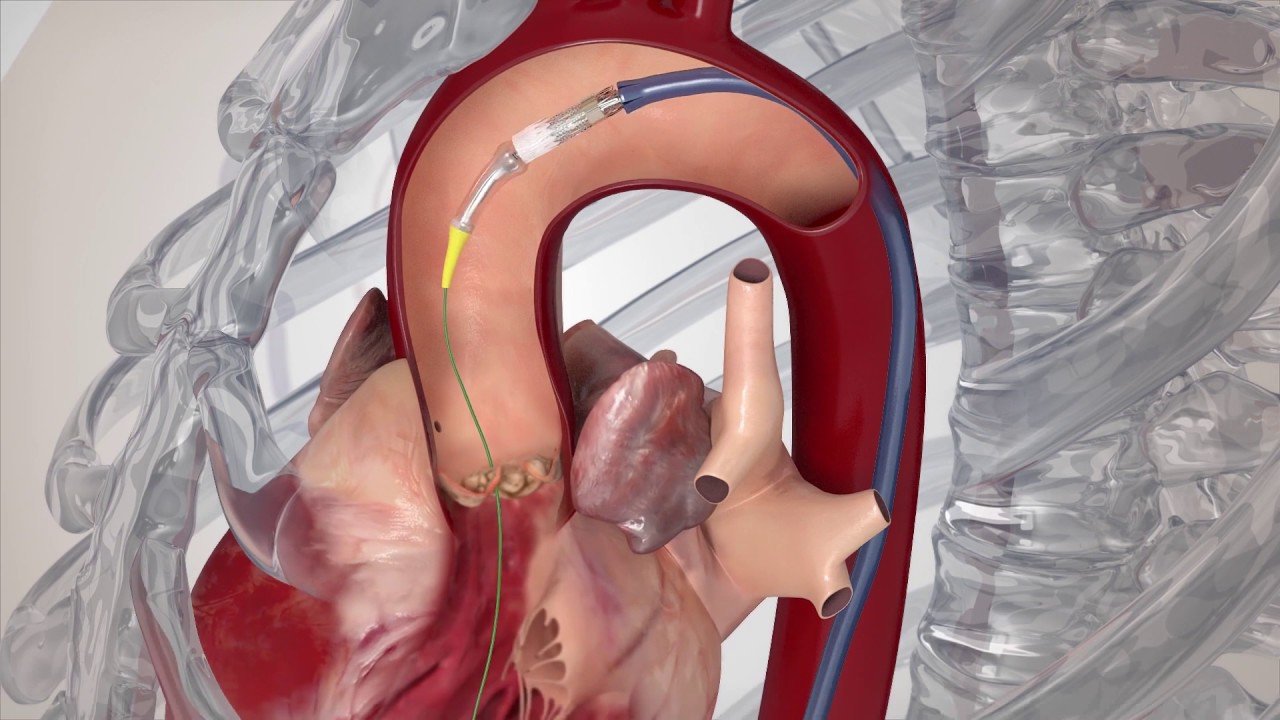
Transcatheter Aortic Valve Replacement (TAVR) is an established treatment modality for patients who are at a high risk or unwilling to undergo open heart valve replacement surgery.
Image credit- youtube.com
Meril Life Sciences has introduced the first ever indigenously designed and manufactured Transcatheter Aortic Heart Valve (TAVR) - Myval. With this launch, Meril, a global medical devices company becomes the First Indian Company to make Transcatheter Aortic Heart Valve Replacement (TAVR) Therapy commercially available on the world stage.
Transcatheter Aortic Valve Replacement (TAVR) is an established treatment modality for patients who are at a high risk or unwilling to undergo open heart valve replacement surgery. TAVR is aminimally invasive procedure in which the Doctor places a replacement valve into the patient’s native diseased valve via a catheter inserted through the femoral artery (large artery in groin). This is an alternative way to replace diseased valves without undergoing traditional open heart procedure (which some patients may not tolerate well).
Commenting on the approval, Sanjeev Bhatt (Vice President-Corporate Strategy, Meril Life Sciences) said, “Meril has always been dedicated towards design and development of novel, clinically relevant, state-of-the-art and best-in-class devices to alleviate human suffering and improve quality of life. The launch of indigenously developed Myval Transcatheter Heart Valve technology is an assertion of this fundamental belief. For us, it is a proud moment to be the first Indian company to commercially make this therapy available in the country. Through the commercialization of this technology, Meril will soon bring the next generation treatment modality to thousands of patients across the country and globally. Making India proud of this achievement.”
Commenting on the approval Dr. P K Minocha (Director, Research & Development, Meril Life Sciences) said,“Since its inception, Meril has played a leading role in developing and introducing innovative medical technologies. The Myval-TAVR technology has been developed after 6 years of extensive research and is backed by robust bench testing, pre-clinical and clinical data. We are committed to take this technology to over 100 countries and benefit thousands of patients across the globe."
Dr. Ashok Seth, Chairman of Fortis Escorts Heart Institute, New Delhi and Head, Cardiology Council of Fortis Group of Hospitals said:
As the Principal Investigator of the clinical trial of MyvalTranscatheter Aortic Heart Valve, it is heartening to note that the device has been proven to be safe and effective as required by the regulatory standards of India. The development of this valve, which is implanted non-surgically through a catheter is also a great achievement for the ‘Made in India’ initiative that such an important R&D has happened from India in a field which till now has been dominated by only American devices. The regulatory approval of the indigenous Myval provides genuine hope for thousands of patients with Aortic Stenosis in India and Asia, who were considered risky for surgery and can now undergo safer Transcatheter Aortic Valve Replacement through non-surgical catheter-based treatment.
Dr.Samin K. Sharma (Chairman – Eternal Hospital, Jaipur, Rajasthan; Director, Clinical and Interventional Cardiology and President, Mount Sinai Heart Network, New York, USA) said:
We have been performing TAVR procedures in USA for over 8 years and it’s heartening to know that Myval-TAVR is a Made in India technology, now going to be available for treatment of Indian population. As the Chairman of MyVal-1 pivotal clinical study in India, I feel excited with possibility of treating patients with aortic stenosis who are not able to have surgery or for whom surgery might be a risky decision.
The Myval TAVRtechnology has been designed and developed by Meril Life Sciences, headquarteredin Vapi, Gujarat,India and has recently received approval for use by Central Drugs Standard Control Organization (CDSCO). The approval was received on the basis of successful results from a clinical trial done in India.All patients are doing well post procedure and during follow-up. This novel Myval technology is associated with Zero new pacemaker implantation rates post procedure (which is an important benefit for the patient already treated for valve replacement). Pacemaker is an additional device that may be placed post TAVR procedure.
TAVR is increasingly becoming a preferred alternative for surgical valve replacement. Faster recovery time and associated clinical benefits for the patients are some of the reasons for this innovative technology’s growing adoption worldwide.