WHO study credits Indian temp proof vaccine for reducing Meningitis in Africa
February 26, 2014 | Wednesday | News | By Rahul Koul Koul
WHO study credits Indian temp proof vaccine for reducing Meningitis in Africa
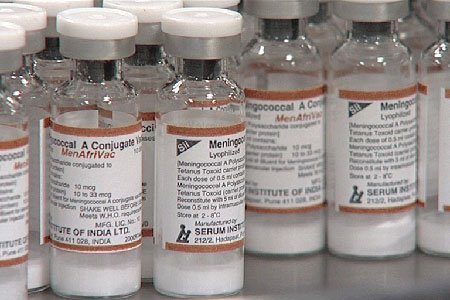
MenAfriVac stayed viable even in ambient temperatures up to 39°C (102.2°F), according to a study published online in the journal 'Vaccine'
For more than 100 years, sub-Saharan Africa has suffered from meningitis A epidemics that claim thousands of lives. In 1996 and 1997, an epidemic killed more than 25,000 people and sickened 250,000. Those who survive often suffer from deafness, epilepsy, loss of limb, and mental retardation.
The MenAfriVac vaccine, which is manufactured by Serum Institute of India, Is already saving lives along Africa's meningitis belt. Since 2010, more than 150 million people aged 1 to 29 years in 12 countries have received the vaccine with not a single group A meningitis case identified in the vaccinated populations. By the end of the 2013 epidemic season, the number of meningitis cases in the belt was the lowest in ten years, a decrease attributed to the introduction of MenAfriVac. By 2016, the vaccine initiative aims to have reached more than 250 million people across the 26 countries in the meningitis belt.
Established in 2001, the Meningitis Vaccine Project is a partnership between PATH and the World Health Organization. Its mission is to eliminate epidemic meningitis as a public health problem in sub-Saharan Africa through the development, testing, introduction, and widespread use of conjugate meningococcal vaccines.
The first mass vaccination campaign conducted in Africa with a vaccine that does not require constant refrigeration succeeded in providing complete coverage while ensuring the vaccine stayed viable even in ambient temperatures up to 39°C (102.2°F), according to a study published online today in the journal Vaccine.
Conducted as part of a ten-day meningitis A vaccination campaign in Benin in November 2012, the study represents a breakthrough not only for the vaccine, MenAfriVac®, but potentially for increasing the efficiency, coverage, and affordability of other lifesaving vaccines as well, especially in remote, hard-to-reach areas where keeping the vaccine cold is difficult. Researchers with Optimize, a now-completed collaboration between the World Health Organization (WHO) and the global health nonprofit PATH, cooperated with the government of Benin to implement the study.
If widely used, this approach could significantly reduce the workloads of health workers, who spend vast amounts of time maintaining the cold chain, and extend vaccines to areas that are so far removed from access to electricity they could never be reached by a cold chain system, the study found.
Additionally, a separate study published in the Bulletin of the World Health Organization on the economic benefits of this approach found that the costs of administering the MenAfriVac vaccine without keeping it cold could drop by 50 percent.