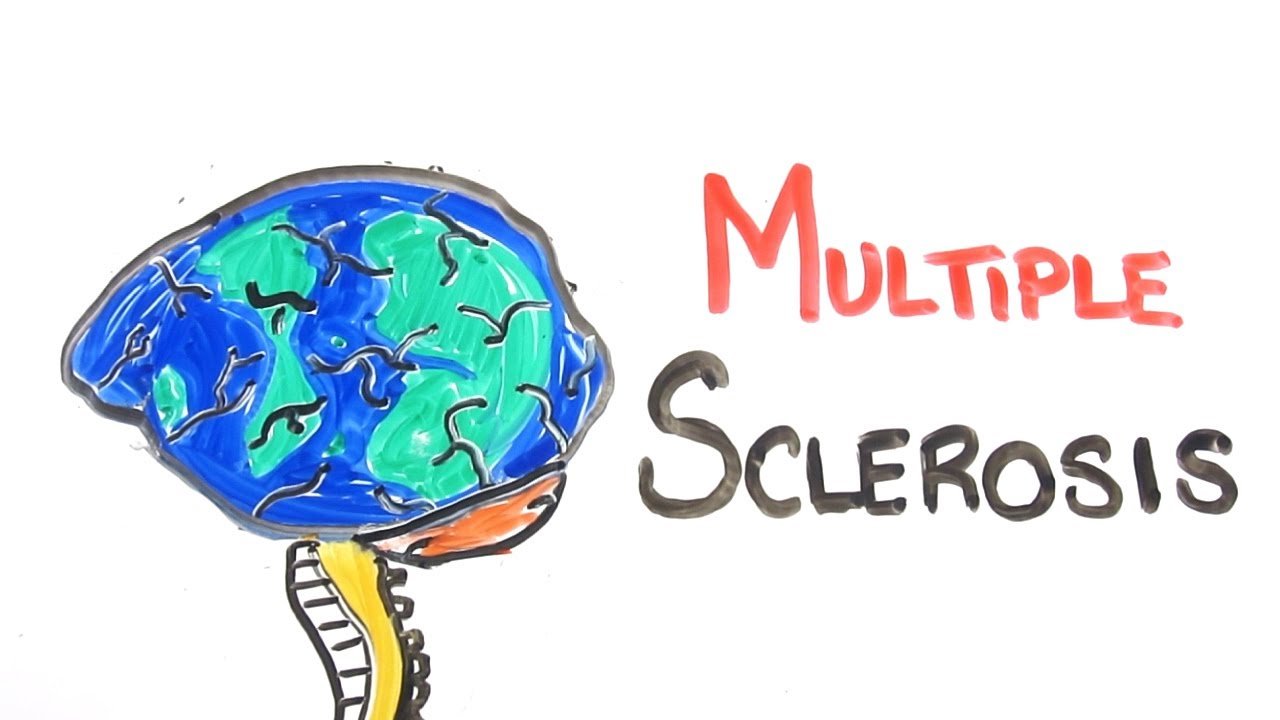
Novartis’ Mayzent gets FDA nod to treat multiple sclerosis
March 27, 2019 | Wednesday | News
Mayzent® (siponimod) is the first and only treatment specifically approved for patients with active secondary progressive multiple sclerosis (SPMS) in over 15 years
Novartis has received the US Food and Drug Administration (FDA) approval of Mayzent® (siponimod) for the treatment of adults with relapsing forms of multiple sclerosis, including secondary progressive multiple sclerosis (SPMS) with active disease, relapsing remitting multiple sclerosis (RRMS) and clinically isolated syndrome (CIS). SPMS is a debilitating form of multiple sclerosis (MS) characterized by progressive and irreversible neurological disability.
Mayzent is expected to be available in the US in approximately one week. Patients will not require a first dose observation (FDO, cardiac monitoring upon initiation) unless they have certain pre-existing cardiac conditions.
"One of the most important aims of MS treatment is delaying disability progression and preserving cognition," said Paul Hudson, Chief Executive Officer, Novartis Pharmaceuticals.
"With Mayzent, SPMS patients with active disease will have access to the first effective oral therapy directed towards disease progression, even when MS transitions to a stage where deterioration is less dependent on the usual relapse activity. Mayzent is a testament to the Novartis mission to reimagine medicine. We are delighted that our ongoing commitment to stop MS has led to a much awaited treatment for these patients in need."
This approval will stimulate a conversation between patients and healthcare professionals about disability progression after relapsing remitting MS and its early management."
Approval is based on the Phase III EXPAND trial, the largest controlled clinical study of SPMS patients, showing Mayzent significantly reduced the risk of disease progression, including impact on physical disability and cognitive decline.
Novartis is committed to bringing Mayzent to patients worldwide, and additional regulatory filings are currently underway with other health authorities outside the US. Regulatory action for Mayzent in the European Union is anticipated in late 2019, with additional regulatory action anticipated in Switzerland, Japan, Australia and Canada this year.