Medical-grade smartwatch CardiacSense receives approval from US FDA & CDSCO
February 16, 2023 | Thursday | News
CardiacSense is now selling in over 40+ countries across the globe including India
Medtech solutions provider Xplore Health, with presence in Pune, Mumbai, Delhi and Bengaluru, has received approval from the Central Drug Standard Control Organisation (CDSCO) for its aspirational medical watch CardiacSense. The approval came ahead of its commercial launch in collaboration with Israeli medtech company CardiacSense Ltd.
The product has already been approved by US Food and Drug Administration (FDA) and is the only medical watch to demonstrate accuracies higher than FDA set thresholds, making it one of the only approved medical watch. With its approval from CDSCO, the CardiacSense will be available in the Indian markets as well.
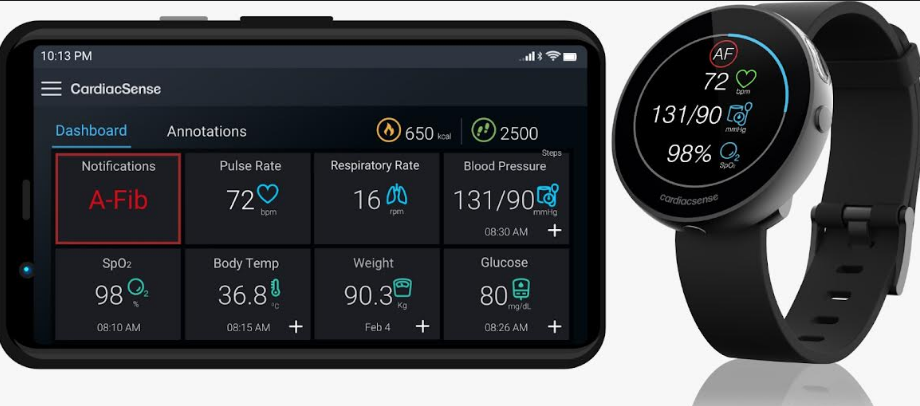
CardiacSense technology is designed to ensure that one is able to continuosly monitor their vital signs and whenever there is a notification raised by watch for increased or decreased heart rates or for arrhythmias, the watch prompts the user to take an ECG and share it instanly with the doctor for immediate advise.
It is one such device that will enable doctors and hospitals to monitor patients 24/7 who have recently undergone major surgeries or those suffering from chronic heart disease or organ failure.
Having received approval from India, CardiacSense is now available in over 40 countries, including the US, all European countries, Australia, New Zealand, and many other South American countries.