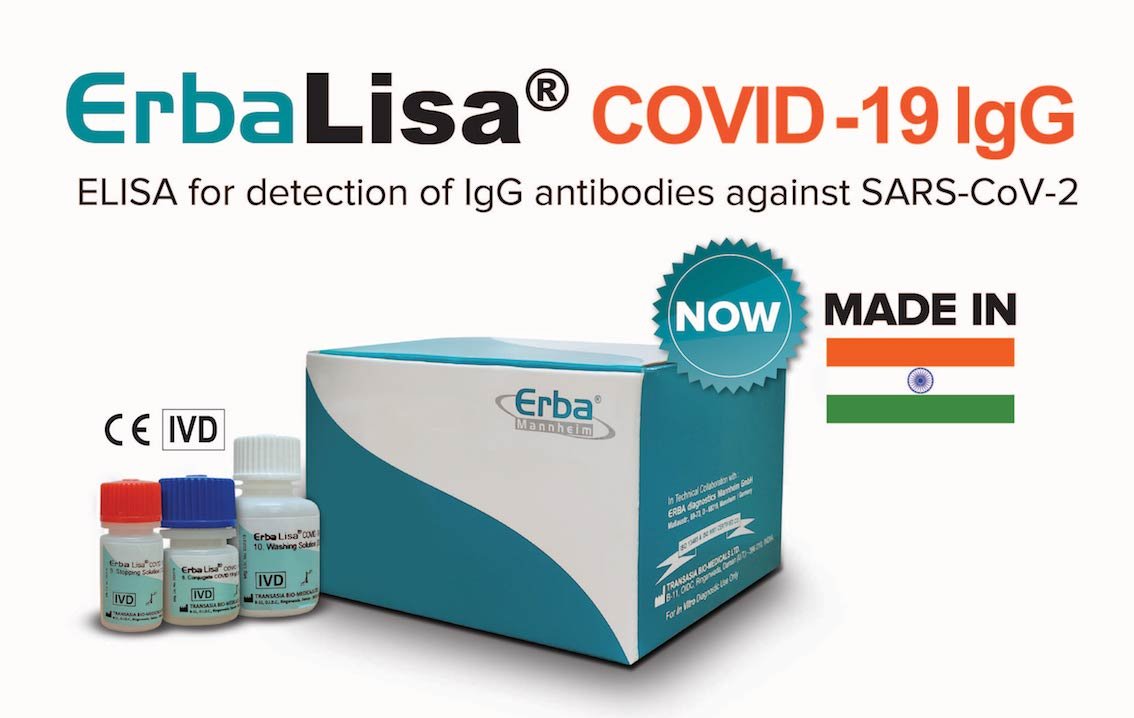
Transasia unveils indigenous ErbaLisa COVID-19 ELISA test kits
August 20, 2020 | Thursday | News
Factory has been set up at Andhra Pradesh MedTech Zone in Visakhapatnam
Mumbai based Transasia Bio-Medicals Ltd., India’s leading IVD company has launched “Made in India” ErbaLisa COVID-19 IgG ELISA-based antibody test kits for detection of COVID-19.
Designed and developed in India, the ErbaLisa COVID-19 IgG ELISA kits are a perfect solution to fight the current scenario of the pandemic in the country. The kits have been validated for a proven sensitivity and specificity of >99% and are suitable for use on all widely available semi and fully automated ELISA systems.
“For the last 40 years, Transasia has been meeting the needs for quality, diagnostic solutions that are accessible and affordable. Very early on, we identified that Manufacturing in India is the only way forward to reduce the dependency on imports and make India truly ATMA NIRBHAR. Our ErbaLisa COVID-19 IgG ELISA kits are a result of dedicated efforts of our R&D team in India. We are proud to remain committed to ATMA NIRBHAR BHARAT by manufacturing these kits in India”, said Suresh Vazirani, Chairman and Managing Director, Transasia-Erba group.
“We are thankful to ICMR and NARI for acknowledging our efforts in developing the COVID-19 testing kits. This approval has come at the right time, as India ramps up its testing capacity and inches towards normalcy. Manufacturing these kits in India, will help us further offer affordable kits that are accessible to the citizens of India and contribute to the government’s strategy of test, treat and track”, added Ravi Kaushik, CEO, Transasia Bio-Medicals Ltd.
ErbaLisa COVID-19 IgG kit has been evaluated and approved by National AIDS Research Institute (NARI), Pune, and is included in the ICMR’s list of validated COVID-19 IgG antibody tests. The kits have also approved by CE IVD. The kit offers the following advantages:
- Sample required for test – Blood Serum/Plasma
- Assay time – 90 min
- Simplified protocol with In-Well Sample Dilution
- Ready to use reagents
- Semi-quantitative result
- > 99% sensitivity and specificity
- Adaptable to automation
“The government has put in a lot of efforts to slow down the surge in the spread of the infection in India. As a company that is truly Indian by heart, we are proud to contribute to the fight against COVID-19. This kit is made in India for the people of India and is dedicated to all COVID heroes who have been relentlessly, ensuring the health of the nation, often putting their own health at risk,” concluded Anil Jotwani, Sr. President, Sales & Marketing, Transasia Bio-Medicals Ltd.
Transasia’s R&D team based in Mumbai, has been working round-the-clock to develop the kit and evaluate it on a large number of population belonging to different demographics in India. The high sensitivity and specificity offered by the MADE IN INDIA ErbaLisa COVID-19 IgG kits can help policy makers plan and implement appropriate public health interventions for prevention and control of the disease. These ready-to-use kits can test up to 96 samples and give results in a couple of hours.