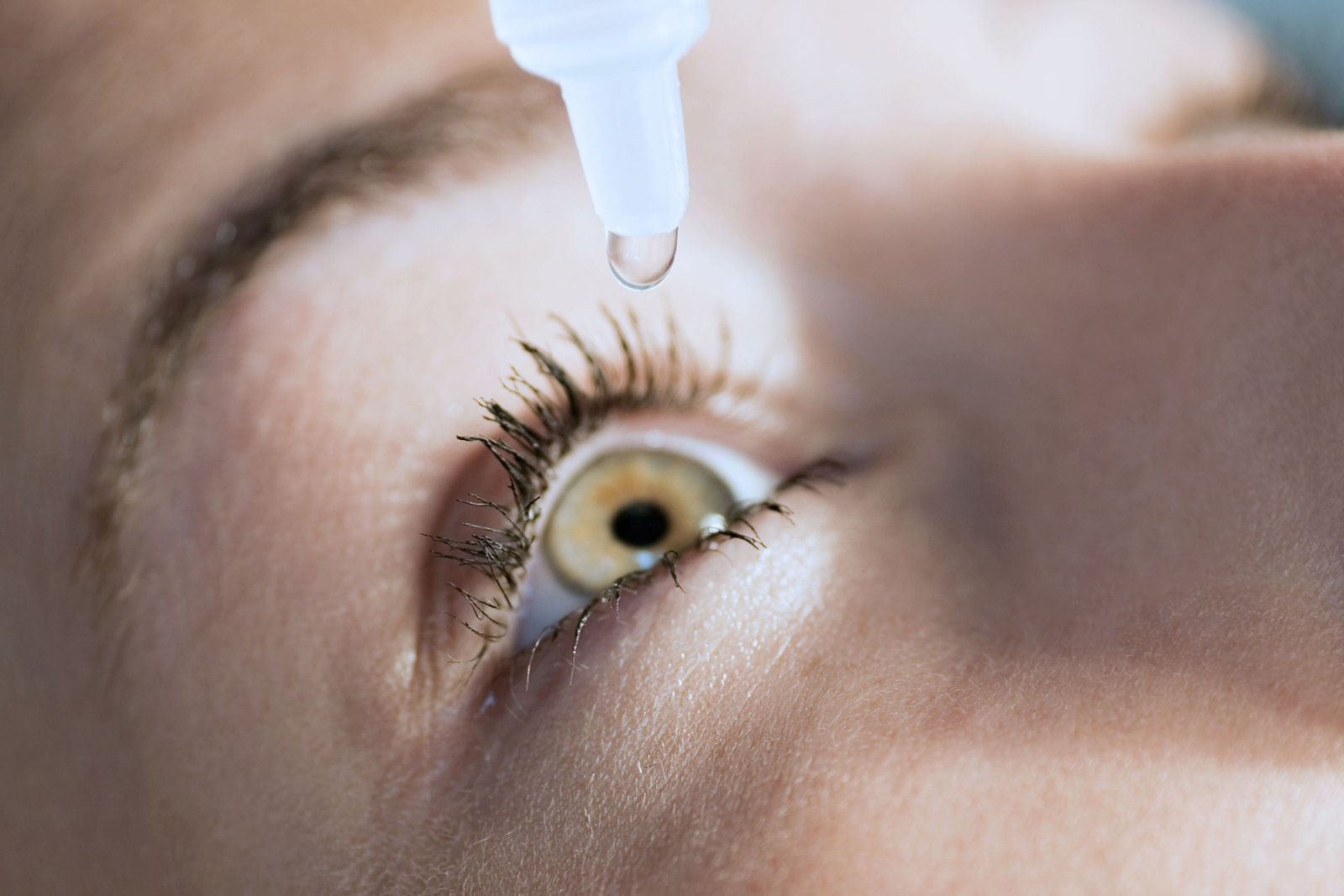
Entod Pharma receives regulatory approval for Presbyopia eye drops
April 20, 2024 | Saturday | News
Clinical trials have demonstrated PresVu's efficacy in enhancing close-up vision within minutes of application
image credit- shutterstock
Entod Pharmaceuticals marks a significant milestone with the approval of PresVu, a revolutionary Treatment for presbyopia, by the Subject Expert Committee (SEC) of the Central Drugs Standard Control Organisation (CDSCO).
PresVu emerges as the first-of-its-kind eye drop in India designed to alleviate the need for reading glasses among individuals grappling with presbyopia, a prevalent age-related vision impairment affecting millions.
Presbyopia, characterised by blurred near vision due to the decreased flexibility of the eye's natural lens, primarily impacts individuals aged 40 and above.
With PresVu, Entod Pharmaceuticals introduces a game-changing intervention, offering swift relief and temporary correction of vision problems associated with presbyopia. Leveraging innovative dynamic buffer technology developed through their DSIR-approved R&D facility, PresVu eye drops adjust rapidly to tear pH, ensuring sustained efficacy and safety for long-term use.
The company completed development of these eye drops in late 2022 after which it was subjected to clinical testing in India. Phase 3 clinical trials carried out in India and the US have demonstrated PresVu's efficacy in enhancing close-up vision within minutes of application, with effects lasting up to six hours. This groundbreaking formulation holds immense promise for individuals aged 40 to 55 with mild to intermediate presbyopia, providing a viable alternative to traditional interventions like reading glasses.