Sanofi receives approval for Dupixent in India for atopic dermatitis treatment
July 11, 2023 | Tuesday | News
Dupilumab is being jointly developed by Sanofi and Regeneron under a global collaboration agreement
Image credit: shutterstock
Sanofi Healthcare India has received marketing authorisation for Dupixent® (dupilumab), the first biologic medicine for the treatment of moderate-to-severe atopic dermatitis in adults whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Dupixent® can be used along with or without topical therapy.
Globally, Dupixent® has transformed the treatment landscape for patients around the world by targeting the type 2 inflammation that underlies the disease, rather than broadly suppressing the immune system.
Anil Raina, General Manager, Sanofi Specialty Care (India) said,“Dupixent® receiving marketing authorization in India is a significant milestone, as we now have the opportunity to offer our first-in-class and best-in-class therapy to treat people living with atopic dermatitis, in India. Approved in the U.S., the European Union, Japan and more than 60 countries for one or more indications other than atopic dermatitis, Dupixent® is the first and only biologic medicine in India that has shown significantly improved disease signs, symptoms, and quality of life measures, for this particularly difficult-to-treat skin condition.”
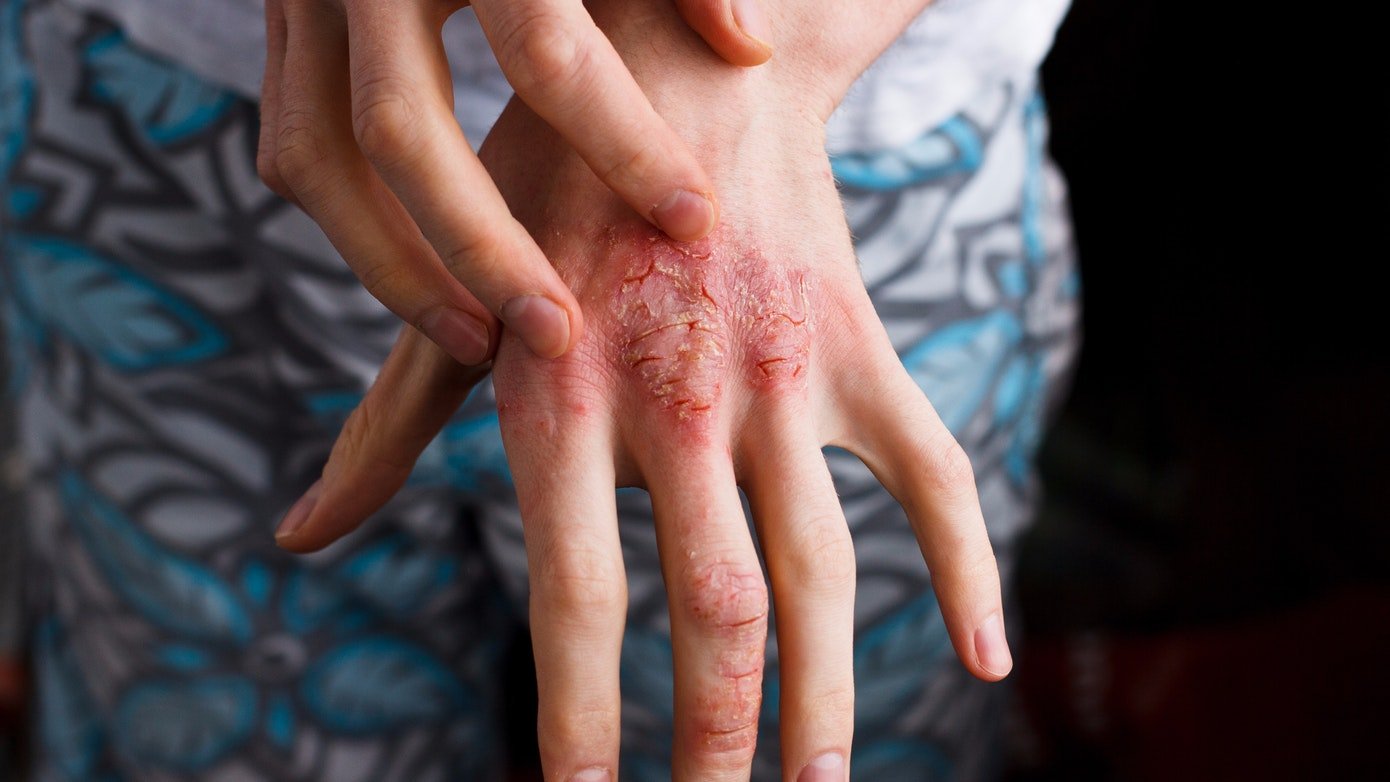
Atopic dermatitis, a form of eczema, is a chronic type 2 inflammatory disease with symptoms often appearing as a rash on the skin. Moderate-to-severe atopic dermatitis is characterized by rashes often covering much of the body, and can include intense, persistent itching and skin dryness, cracking, redness, crusting, and oozing.
With more than 600,000 patients being treated with Dupixent® globally, Dupixent® will soon be available as an option for controlling moderate to severe atopic dermatitis for adults in India.