MicroVention announces FDA premarket approval of new Flow Diverter
January 22, 2020 | Wednesday | News
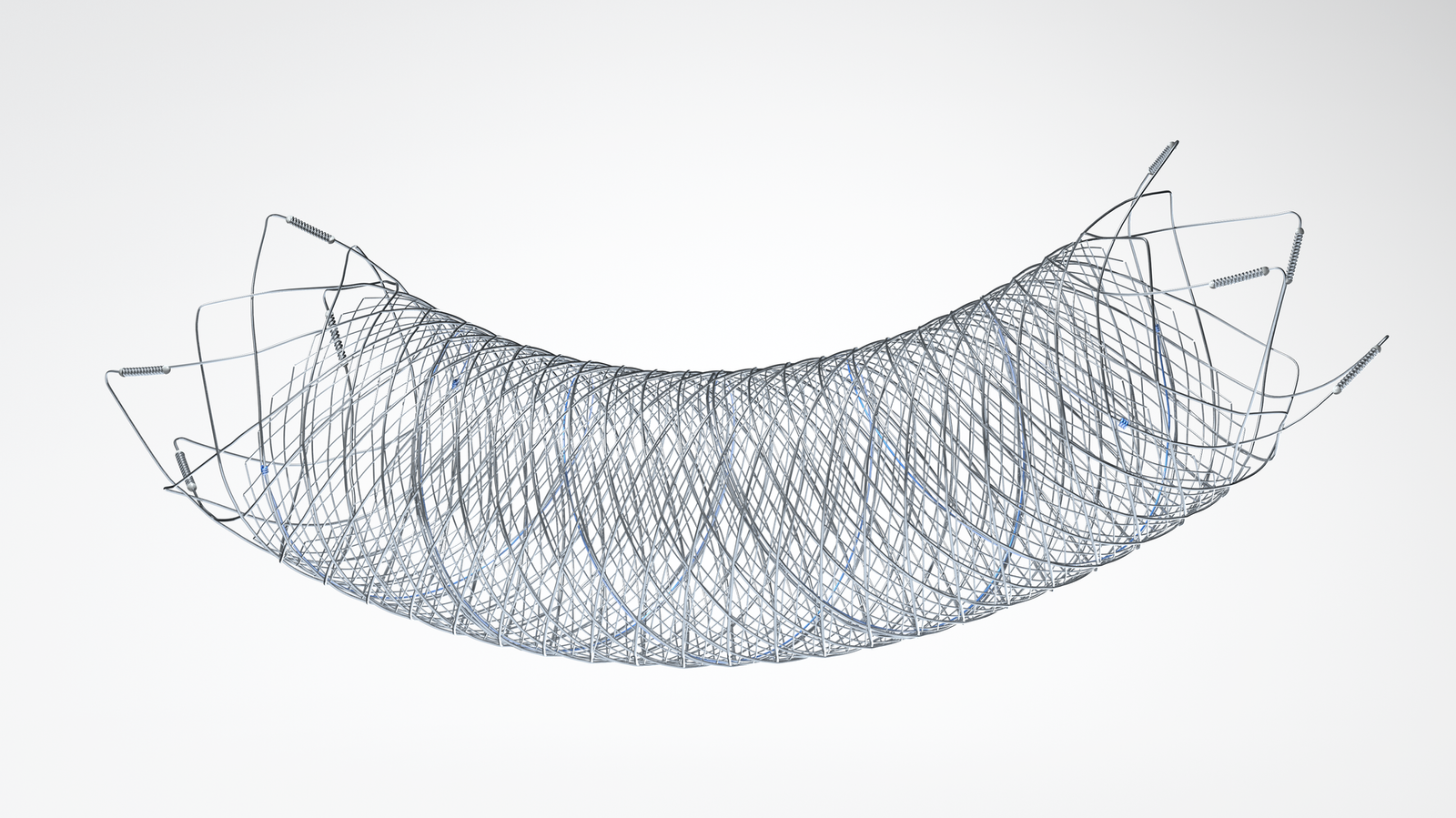
The FRED™ device is the first flow diverter in the U.S. to use a self-expanding braided nitinol mesh
image credit- shuttershock.com
MicroVention, Inc., a U.S. based subsidiary of Terumo and a global neurovascular company announced the FDA Premarket Approval (PMA) for the FRED™ (Flow Re-Direction Endoluminal Device) device for the treatment of brain aneurysms.
The FRED™ device is the first flow diverter in the U.S. to use a self-expanding braided nitinol mesh to help re-direct blood flow and promote aneurysm occlusion. The unique interwoven nitinol design of the FRED™ device allows for smooth delivery to the target aneurysm, as well as reliable opening and vessel wall apposition, resulting in high treatment durability.
Dr. Cameron McDougall, Director of Endovascular Neurosurgery and Professor of Neurosurgery at The Johns Hopkins Hospital, and primary investigator for the FRED™ pivotal trial said, “The FRED™ device represents a step forward in flow diversion technology with the inclusion of the lowest profile delivery platform in the U.S. This advancement will allow physicians to more easily access and treat wide-necked or fusiform aneurysms. The pivotal study shows that a single FRED™ device is safe and effective for use in a variety of aneurysm sizes and locations and expands treatment options for patients.”
Irina Kulinets, PhD, Sr. WW Vice President of Regulatory Affairs, Clinical Research & Quality at MicroVention said, "With our 3rd PMA approval by the Food and Drug Administration in 18 months, MicroVention is proud to introduce the FRED™ flow diversion device to the United States. The FRED™ device helps address a need for a clinically proven flow diverter with simplified delivery. MicroVention is dedicated to the development of novel technologies that improve patient outcomes and quality of life. We are excited to bring the FRED™ device to patients in the U.S. who will benefit from treatment."
The FRED™ device has been CE marked since 2013, safely used in nearly 20,000 procedures, and published in numerous clinical studies around the world. The FRED™ device pivotal study adds new evidence to the large existing body of global clinical data, further demonstrating the safety and effectiveness of the device.