ICMR updates ethical guidelines for application of AI in biomedical research and healthcare
March 24, 2023 | Friday | News
To provide a framework for ethical decision-making in medical AI
The Indian Council of Medical Research (ICMR) has released “Ethical Guidelines for Application of Artificial Intelligence in Biomedical Research and Healthcare, 2023” to ensure ethical conduct and address emerging ethical challenges in the field of Artificial Intelligence (AI) in biomedical research and healthcare.
These guidelines are also meant to provide a framework for ethical decision-making in medical AI during the development, deployment, and adoption of AI-based solutions. The guidelines are intended for all stakeholders involved in research on AI in biomedical research and healthcare, including creators, developers, researchers, clinicians, ethics committees, institutions, sponsors, and funding organisations.
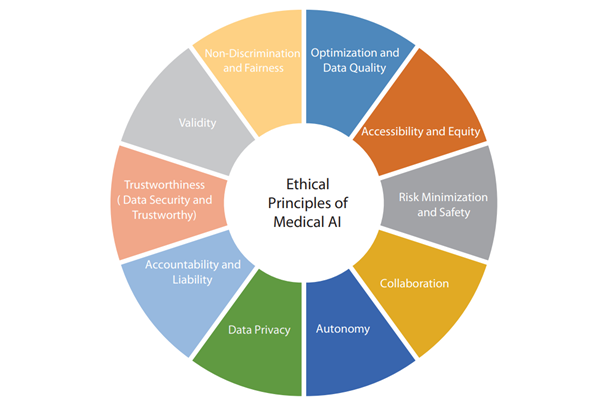
The guidelines include sections on ethical principles, guiding principles for stakeholders, an ethics review process, governance of AI use, and informed consent. They have been developed through extensive discussions with experts and ethicists. The guidelines are a living document and will be updated as ethics in AI evolve.
"The adoption of AI technology in healthcare is growing in India. However, AI as data-driven technology has many potential ethical challenges which include algorithmic transparency and explainability, clarity on liability, accountability and oversight, bias and discrimination", said Dr Rajiv Bahl, Director General, ICMR.
ICMR has formulated ethical guidance documents from time to time for promoting ethical and high quality research in India. The most recent version of ICMR’s National Ethical Guidelines for Biomedical and Health Research involving human participants, was released in 2017.