Bone Marrow-derived Human Mesenchymal Stem Cell Production in Corning® HYPERStack® 36-layer Cell Culture Vessels
October 31, 2022 | Monday | Reports/white papers | By Hilary Sherman and John Shyu Corning Incorporated, Life Sciences Kennebunk, ME USA
Mesenchymal stem cells (MSCs) are multipotent cells that have recently generated significant interest for cellular therapy applications
MSCs have the potential to differentiate into other mesenchymal tissue lineages such as adipocytes, osteocytes, and chondrocytes1. Additionally, they are known to secrete trophic factors that can play an important role in immunoregulation1.
Although MSCs can be isolated from different tissue sources, bone marrow-derived MSCs are commonly studied due to their ease of access and achievable therapeutic dosage (2 x 106 cells/kb of body weight)2,3. Here, we demonstrate the utility of the Corning HYPERStack 36-layer cell culture vessel as a tool to meet the growing demand for expanding bone marrow-derived MSCs to relevant scale for clinical application workflows. The utilization of gas permeable film technology provided in the spatial footprint of a traditional stacked cell culture vessel enables Corning HYPERStack cell culture vessels to provide up to 5X the surface area in the same spatial footprint for expansion of adherent cell types, such as MSCs. Our results show that over 870 million human bone marrow-derived MSCs can be obtained from a single HYPERStack 36-layer cell culture vessel. Furthermore, harvested cells demonstrated high viability and expressed characteristic surface markers of human bone marrow-derived MSC identity.
Materials and Methods
Human bone marrow-derived MSCs (RoosterBio Cat. No. MSC- 1M-5XF) were thawed into T-175 flasks (Corning Cat. No. 431080) containing RoosterNourish™-MSC-XF (RoosterBio Cat. No. KT-016) per vendor’s recommended protocol. Upon achieving 90% confluence, cells were harvested with TrypLE™ Express Enzyme (ThermoFisher Cat. No. 12604021) and centrifuged at 200 x g for 10 minutes. Cells were re-plated in Falcon® 875 cm2 Multi-Flasks (Corning Cat. No. 353144) at a density of 3 x 103 cells/cm2. After five days of culturing in a humidified, 5% CO2 incubator at 37°C, cells were harvested as previously described and seeded into
pre-warmed Corning HYPERStack 36-layer cell culture vessels (Corning Cat. No. 20036) at 3 x 103 cells/cm2. It is recommended to pre-warm the Corning HYPERStack 36-layer cell culture vessel at 37°C to prevent any temperature gradients during the seeding process. Human bone marrow-derived MSCs were expanded in the HYPERStack cell culture vessels for five days, then Cells were harvested and assessed for yield and viability. The expansion from cryogenic vial thaw through HYPERStack 36-layer cell culture vessel expansion was repeated three independent times. To confirm MSC identity, approximately 1 x 107 cells were stained (BD Biosciences Cat. No. 562245) per vendor protocol and CD surface antigens assessed via flow cytometry.
Results and Discussion
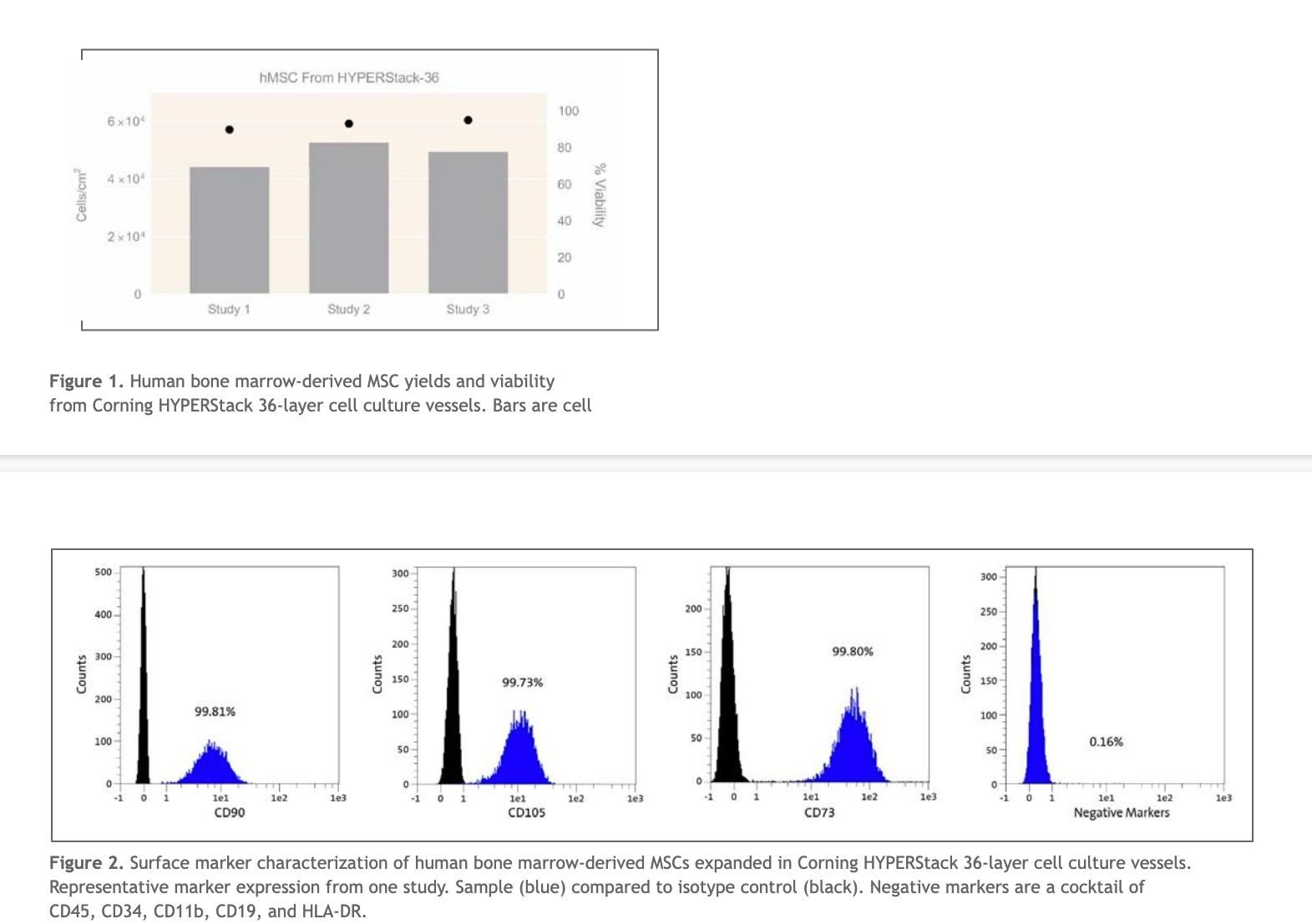
Human bone marrow-derived MSC densities ranging from 4.4 x 104 to 5.2 x 104 cells/cm2 were achieved after 5 days of culture in Corning HYPERStack 36-layer cell culture vessels (Figure 1). The average of all three studies resulted in a total MSC yield of more than 8.7 x 108 cells per HYPERStack 36-layer cell culture vessel. For therapeutic application workflows, it is essential to recover MSCs that have high viability and express appropriate surface markers4. In these studies, MSCs collected from HYPERStack 36-layer cell culture vessels showed greater than 90% average viability (Figure 1). The International Society for Cellular Gene Therapy (ISCT) has defined the minimal criteria for human bone marrow-derived MSC quality as expressing >95% of CD105, CD73, and CD90 and lack of expression (<2%) of typical hematopoietic markers CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR surface molecules4. Human bone marrow-derived MSCs recovered from HYPERStack 36-layer cell culture vessels were characterized via flow cytometry, and displayed greater than 99% expression of CD90, CD105 and CD73 while expressing less than a half a percent of negative markers (CD45, CD34, CD11b, CD19, and HLA-DR).
Conclusions
The growing number of clinical trials involving stem cells used for therapeutic applications has led to an increase in demand for tools and technologies that enable efficient scale-up of stem
cells, without compromising cell viability and cell health. Here we demonstrate that the Corning® HYERStack® 36-layer cell culture vessels offers an effective method for expanding large quantities of human bone marrow-derived MSCs that maintain high viability and appropriate surface marker characterization.
References
- Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning and Stem Cells (2004) 6.4:369-374.
- Musiał-Wysocka A, Kot M, and Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant (2019) 28.7:801-812.
- Hassan M, et al. Large-scale expansion of human mesenchymal stem cells. Stem Cells International (2020).
- Robb KP, et al. Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy (2019) 21.3:289-306.