Covidien unveils its new product
October 29, 2014 | Wednesday | News | By BioSpectrum Bureau
Covidien unveils its new product
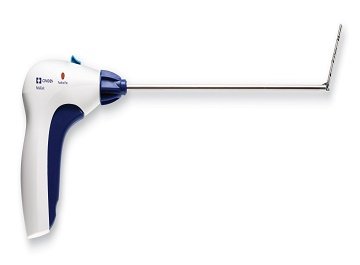
The device has received FDA clearence (Photo: Business wire)
Covidien has announced the launch of ReliaTack articulating reloadable fixation device, the first and only of its kind for laparoscopic (minimally invasive) hernia repair.
"Developed with valuable input from surgeons, the ReliaTack device, combined with our recently-launched Symbotex composite mesh, benefits patients while helping drive greater operating room efficiency and economic benefits. The ReliaTack fixation device overcomes numerous physical and ergonomic challenges in ventral hernia repair by allowing a wide variety of angles to more effectively position and secure hernia mesh,"said Mr Rob Claypoole, vice president and general manager, Hernia, Covidien.
The ReliaTack device has received FDA 510(K) clearance.
"Covidien's proven tack design helps surgeons ensure that the mesh will stay in place, one of the key concerns of hernia repair surgery. By offering interchangeable tack reload sets, ReliaTack helps to eliminate much of the waste associated with non-articulating devices," said Mr Matt Cohen, vice president, R&D Covidien.