IPA urges USTR for removal of India from Priority Watch List
February 11, 2019 | Monday | News
To facilitate the Special 301 Review, USTR invites written submissions. The period for submissions closed on 7 February 2019.
Representative Image
IPA has drawn the attention of the USTR to the rapid progress made by India on a number of fronts which were cited by the USTR while placing India on the Priority Watch List in 2018.
The 2018 Report had asserted that ‘India has yet to take steps to address longstanding patent issues’, among which were the ‘long timelines for receiving patents’. IPA has pointed out that a number of steps have been taken by the Patent Office to augment manpower and streamline procedures which have resulted in the transformation of the Patent Office. As a result, patent examination time has been brought down drastically. Patents pending examination have come down from 204,177 as on 31 March 2017 to 127, 881 as on 31 December 2018. India now examines trademark applications in about one month and registrations are completed in a year or less.
Commenting on this development, D G Shah, the Secretary General of the IPA said, ‘India now has one of the lowest examination times for trademark registration in the world and the indications are that patent examination backlog will be eliminated in about two years. Processing delays are no longer a concern and we hope that the USTR will take note of this.’
The more substantive concerns of the U.S. pharmaceutical industry stemming from some provisions in India’s IP Laws are:
One that is particularly troublesome to innovator companies is Section 3(d) which prohibits the grant of ‘evergreening’ patents. These are additional patents for a drug with no therapeutic benefit and serve only to increase the term of patent monopoly. Consequently, the availability of affordable generics is delayed and assures innovators of an extended period of monopoly pricing.
India also does not provide a term of data (market) exclusivity for a new drug, quite apart from the monopoly conferred by a patent for it.
Compulsory licensing of patent permitting generic manufacturers to develop and market an affordable generic version when the price of the innovator drug is very high is another major irritant to innovator companies.
The extraordinarily high prices of new drugs is a cause of considerable concern globally’, says D G Shah. ‘Even developed countries in Europe such as Netherlands and Switzerland are exploring the possibility of compulsory licensing as a means to control prices of new drugs’.
IPA has strongly defended India’s patent law which is TRIPS-compliant and strikes a balance between the need to assure innovators of profits for their useful inventions and safeguarding public health.
Each year, the Office of the United States Trade Representative (USTR) conducts a Special 301 review to identify countries that deny adequate and effective protection of intellectual property rights (IPR) or deny fair and equitable market access to U.S. persons who rely on intellectual property protection. Based on this review, the United States Trade Representative determines which of these countries are identified as Priority Foreign Countries. Once a country is identified as a Priority Foreign Country, the USTR must open a Section 301 investigation, which may lead to trade sanctions.
In addition, USTR has created a “Priority Watch List” and “Watch List” to assist the U.S. Administration in pursuing the goals of the Special 301 provisions. Placement of a trading partner on the Priority Watch List or Watch List indicates that particular problems exist in that country with respect to IPR protection, enforcement, or market access for persons that rely on intellectual property protection. Trading partners placed on the Priority Watch List are the focus of increased bilateral attention concerning the problem areas.
To facilitate the Special 301 Review, USTR invites written submissions. The period for submissions closed on 7 February 2019. The USTR will hold a public hearing for those who wish to appear before it as a witness and make oral submissions on 27 February 2019. The Special 301 Report will be released on or about 26 April 2019.
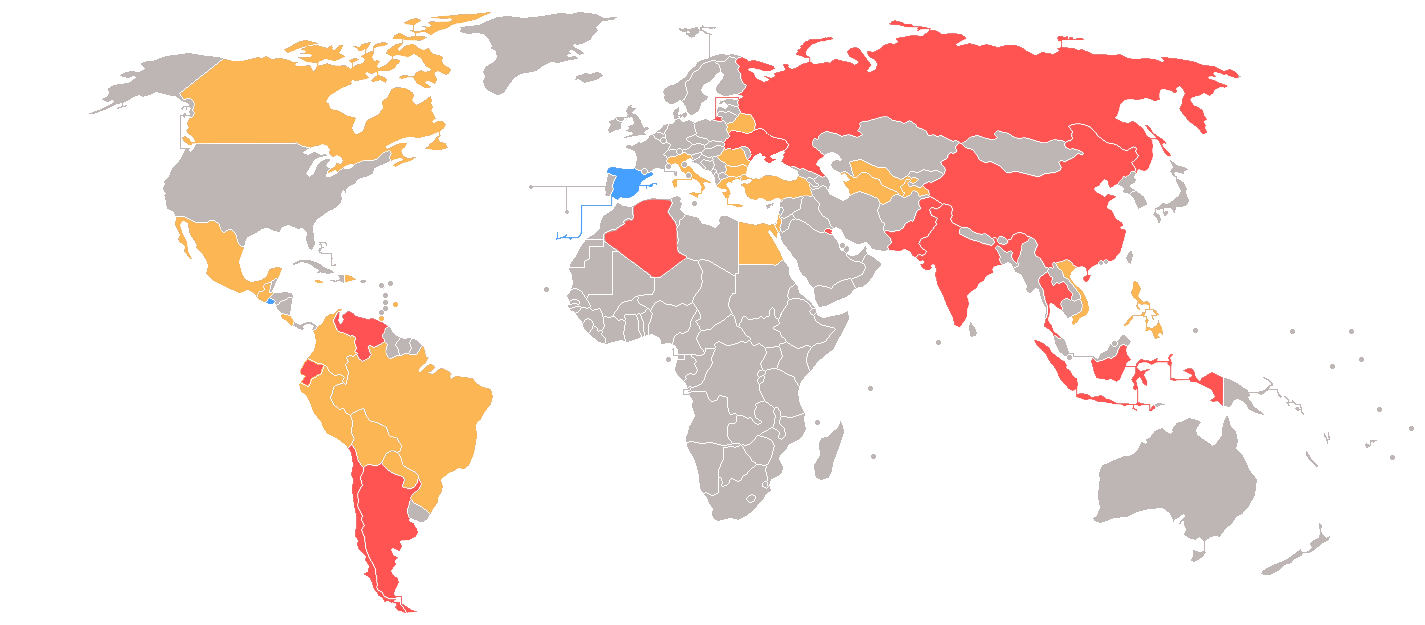
India is one of the twelve countries placed in the Priority Watch List consequent to the 2018 Special Review. The other countries are Algeria, Argentina, Canada, Chile, China, Colombia, Indonesia, Kuwait, Russia, Ukraine and Venezuela.
The Indian Pharmaceutical Alliance (IPA) is an association of twenty-two pharmaceutical companies which collectively account for over 85 per cent of the private sector investment in pharmaceutical research and development, more than 80 per cent of India’s pharmaceutical exports and over 50 per cent of the domestic pharmaceutical market.