US FDA clears Qure.ai's chest X-ray solution as potential indicator for identifying heart failure risk
September 29, 2023 | Friday | News
The clearance of qXR-CTR marks the company's 12th FDA-cleared algorithm
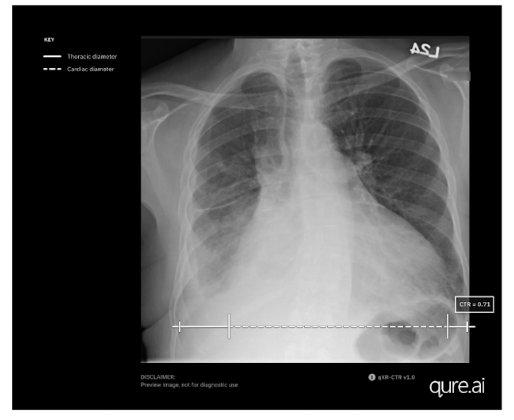
Mumbai-based startup Qure.ai has announced US Food and Drug Administration (FDA) clearance for measuring the cardiothoracic ratio (CTR) utilising its artificial intelligence (AI)-enabled chest X-ray solution, qXR-CTR.
The qXR-CTR is a deep-learning-based computer vision algorithm designed for use by physicians in all inpatient and outpatient settings, to automate the CTR assessment on chest radiographs (CXR). Precisely, it measures the ratio of the maximum transverse diameter of the heart to the maximum inner transverse diameter of the thoracic cavity, providing the most accurate indicator of cardiomegaly on plain film.
This cutting-edge technology leverages artificial intelligence algorithms to deliver precise and efficient results, saving time for physicians and improving diagnostic accuracy.
The clearance of qXR-CTR marks the company's 12th FDA-cleared algorithm, solidifying its position as an industry leader. Previous FDA-cleared findings by Qure.ai include Endotracheal Tube location, Tracheostomy tube location, Pneumothorax, and Pleural Effusion identification for CXR; qER for intracranial haemorrhage detection on head CT scans, and qER Quant for quantifying critical abnormalities on head CT scans.
Heart failure is a complex, life-threatening cardiac condition often misdiagnosed due to its nonspecific symptoms. With qXR-CTR now being cleared by the FDA, Qure's chest X-ray algorithm can be deployed to jointly screen for increased cardiothoracic ratio (CTR) and Pleural effusion along with other radiographic markers associated with the presence of heart failure.