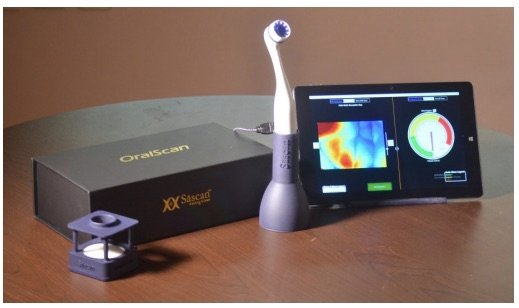
Sascan Meditech launches hand held oral cancer screening tool
October 30, 2020 | Friday | News
Oral Scan is a Make in India initiative with seed funding from the scheme National Initiative for Developing and Harnessing Innovations (NIDHI) of the Department of Science & Technology (DST), Govt of India
Sascan Meditech, incubated at TiMED, the Technology Business Incubator of Sree Chitra Tirunal Institute for Medical Sciences & Technology (SCTIMST), Thiruvananthapuram has launched OralScan, a hand held imaging device for screening, detection and biopsy guidance of oral cancer. Oral Scan is a Make in India initiative with seed funding from the scheme National Initiative for Developing and Harnessing Innovations (NIDHI) of the Department of Science & Technology (DST), Govt of India.
Oral scan was designed and developed entirely in India and supported by Biotechnology ignition grant of Biotechniology Industry Research Assistance Council (BIRAC), INVENT (DST) and Kerala Start Up Mission. The company recently received investment from Unicorn India Ventures.
According to Dr Subhash Narayanan, CEO, Sascan, various studies have demonstrated that oral screening technique is not very reliable and often oral potentially malignant lesions (OPMLs) go undetected in the early stages. Even experienced clinicians find it difficult to locate the optimal site for a biopsy based on conventional oral examination. It is in this context that the OralScan, a handheld imaging device developed by Sascan for screening, detection of OPMLs and biopsy guidance becomes relevant. A proprietary software assists the surgeon in taking biopsy from the most appropriate site which is likely to confirm the diagnosis of malignancy.
The device will be marketed at a price of Rs 5.9 lakh. This will be a onetime investment for hospitals and laboratories without any additional costs of consumables.
Sascan has already obtained ISO 13485 certification and CE certification. An Indian patent has been granted for the technology and US patent has been filed. OralScan has also undergone multi-centric trials covering six hospitals across the country.
“This device is expected to have good demand in General Dentistry, Oral Medicine, Oral/Maxillofacial Pathology and surgery,” said S Balram, CEO, Sree Chitra.