Is target of 216.6 Cr jabs ‘Mission Impossible’ for India?
June 02, 2021 | Wednesday | News
The nation currently is suffering from an acute shortage of vaccines, due to which various vaccination centres have had to shut down vaccinations for several days
India opened its doors to foreign companies that manufacture COVID-19 vaccines to come to India on April 13, 2021, those which have been approved for restricted use by US Food and Drug Administration (US FDA), European Medicines Agency (EMA), UK Medicines and Healthcare products Regulatory Agency (MHRA), Pharmaceuticals and Medical Devices Agency (PMDA) Japan or which are listed in the WHO’s emergency use listing (EUL). Prior to this, India was using Covishield manufactured by Serum Institute of India and COVAXIN manufactured by Bharat Biotech to vaccinate the citizens of the nation against COVID-19. On April 12, 2021, the Drug Controller General of India (DCGI) approved the Russian COVID-19 vaccine, Sputnik V. Sputnik V became the first foreign vaccine to receive approval in India.
A tall order
As of June 1, 2021, India has vaccinated a total of 21,83,58,591 citizens, of which 17,06,78,541 have received the first dose and 4,36,78,226 have received the second dose. India has been reeling under the second wave of COVID-19 that has taken the country by storm. Considering the population of India, the figures are measly and the situation grim, with only 3.2 per cent of the population vaccinated. The nation currently is suffering from an acute shortage of vaccines, due to which various vaccination centres have had to shut down vaccinations for several days. To ease access to vaccines the Government of India granted permissions to states for sourcing COVID-19 vaccines from manufactures across the globe.
So far there are 121 COVID-19 vaccine candidates globally, out of which 17 have received approvals in at least one country and the rest are in various phases of clinical trials. In India, Zydus Cadila’s, ZyCoV-D COVID-19 vaccine is currently in the third phase of clinical trials, Biological E Limited has four vaccine candidates: BECOV2A, BECOV2B, BECOV2C, BECOV2D, all of which are in the second phase of clinical trials, Bharat Biotech’s BBV154 vaccine candidate (nasal vaccine) is undergoing phase one clinical trial.
On May 13, 2021, during a media briefing Dr V K Paul, Member, NITI Aayog addressed the vaccine crisis in the country. He didn’t shy away from mentioning certain milestones that India has achieved since the vaccination drive commenced on January 16, 2021. He mentioned that India has administered 13 per cent of the total COVID-19 vaccines administered worldwide and that India was the first nation to reach the 17 crore milestone of vaccines delivered in the shortest time frame globally.
Dr Paul spoke at length about the strategies that GoI has devised along with all the states/UTs. Under the new liberalised strategy the government has made all efforts to enable all foreign vaccine manufacturers to obtain approvals expeditiously. Approved vaccines would be granted import licences within a time frame of one-two days. The centre has de-centralised the procurement policy of vaccines as states wanted flexibility. This is working seamlessly and towards the welfare of the nation. States have been floating tenders in the global market to procure COVID-19 vaccines without any interference from the central government. States have been advised to make dedicated teams work seamlessly with the centre for the vaccination drive so that vaccines are delivered efficiently, reducing roadblocks as much as possible.
No stone unturned for jabs
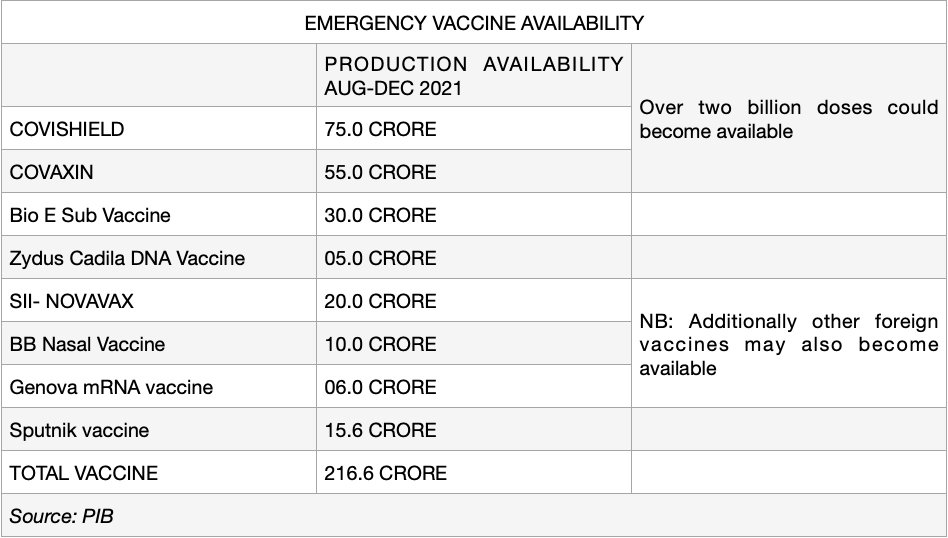
India has an optimistic vision of procuring about 216.6 crore does of COVID-19 vaccines from various manufacturers by December 2021. The Russian Direct Investment Fund (RDIF) , as well, has inked manufacturing contracts with six Indian companies including Hetero Biopharma, Gland Pharma, Stelis Biopharma, Panacea Biotec, Virchow Biotech and Shilpa Medicare, to manufacture more than 850 million doses of Sputnik V.
With an adult population of about 95 million this is very encouraging as this could solve the vaccines woes of our nation and also vaccinate all those who are eligible for the vaccine. Currently, at least one third of the population aged 45 and above have received at least one dose of the vaccine.
The Department of Biotechnology, other departments concerned, Ministry of External Affairs have established contact with Pfizer, Moderna and J&J as well to procure COVID-19 vaccines. J&J in August 2020, had inked an agreement with Biological E to manufacture about 600 doses of the single shot vaccine annually, but there is no clarity when the vaccine will be available. On the other hand Pfizer and Moderna have stated that they will consider India’s offer only in Q3 of 2021. The Indian government is hopeful that these companies will come forward to make their vaccine available in India very soon.
The Indian government is also open to companies coming forward to manufacture COVAXIN, with full support from the government to ramp up manufacturing capacity. Bharat Biotech has been very cooperative it terms of technology transfer for the manufacturing of COVAXIN. Gujarat Biotechnology Research Centre (GBRC), recently established as an autonomous institute under the Department of Science and Technology (DST), along with Ahmedabad-based firms Hester Biosciences (poultry vaccine manufacturer) and OmniBRx have firmed up discussions with Bharat Biotech to scale up the COVAXIN technology and to produce minimum 20 million doses per month. The technology transfer agreement has been finalised. The central government has also provided aid of Rs 65 crore to Bharat Biotech’s new Bengaluru facility which is being repurposed to increase the capacity of vaccine production. Mumbai-based Haffkine Institute has, also, received approval to manufacture COVAXIN, with an annual capacity of around 22.8 crore doses. Haffkine was granted Rs 65 crore from the centre and Rs 94 crore from Maharashtra government to commence operations. The production is expected to commence from 2022 onwards.
216.6 crore doses of COVID-19 vaccine will definitely solve the dearth of vaccines that the nation is currently facing, we need to keep our fingers crossed and hope that all goes to plan. As the pandemic rages on, winning the war against the pandemic is top priority, these vaccines are our shields and weapons. The more we have the better it is. The entire world is facing a vaccine crunch as the supply is finite in nature. Also, vaccine manufactures should consider technology transfers globally, not only in India. COVID-19 has waged a war against mankind, we all are in this together. Time is of the essence right now, vaccine manufacturers should put aside their greed and ambitions and work cohesively to rid the world of the COVID-19 pandemic.
prabhat.prakash@mmactiv.com