Medtronic unveils new transcatheter aortic valve Evolut™ FX+ in India
September 13, 2025 | Saturday | News
Helping more patients benefit from advanced treatment options for structural heart disease
Medtronic, a global leader in healthcare technology, has launched its new transcatheter aortic valve, Evolut™ FX+, in India. The valve is designed to help improve treatment for patients with severe aortic stenosis, especially those who may need future coronary procedures.
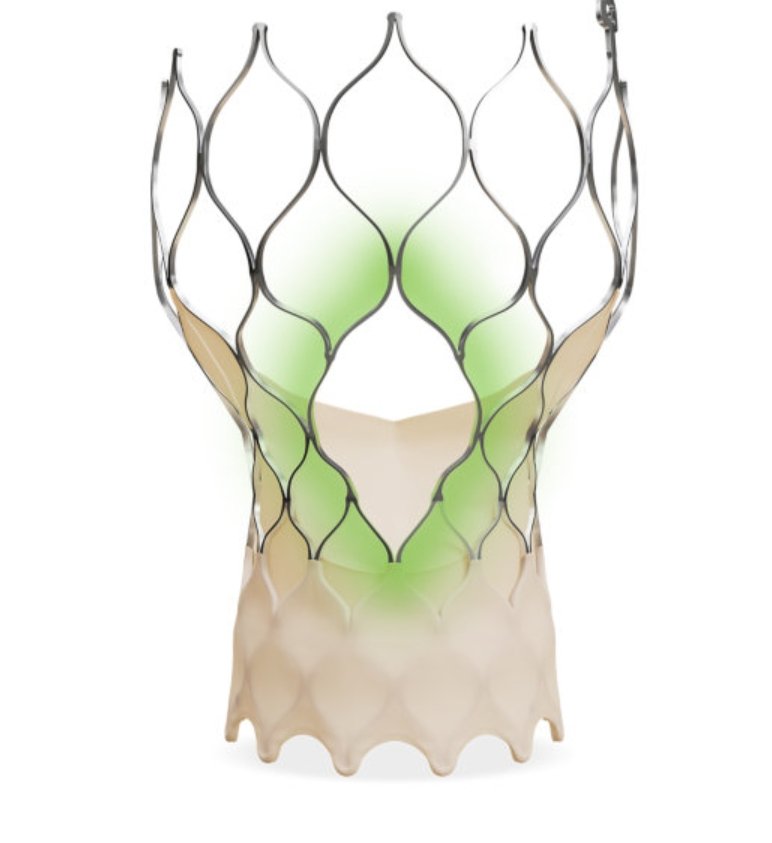
The Evolut FX+ TAVI system facilitates easier coronary access while maintaining the proven performance of the Evolut™ platform, based on CoreValve™ technology. It was approved by the Central Drugs Standard Control Organization (CDSCO) on June 10, 2025, paving the way for its launch in India.
The main innovation of Evolut FX+ is its new frame with bigger windows to reduce frame interference while using guide catheters, especially during complex coronary procedures. The system continues to deliver the key advantages that clinicians have come to expect from previous Evolut valves. The system provides market leading valve performance including durable low gradients, which are important for long-term heart function. This makes it a strong alternative for patients at risk of coronary disease while supporting long-term treatment needs.
The introduction of Evolut FX+ in India marks a significant advancement in transcatheter aortic valve therapy and reinforces Medtronic’s leadership in structural heart innovation. With enhanced coronary access and proven hemodynamic performance, the Evolut FX+ empowers physicians to deliver both immediate procedural success and sustained long-term care. This milestone underscores Medtronic’s steadfast dedication to broadening access to advanced therapies and driving innovation in cardiovascular care and treatment across India.