India Ramps Up HPV Vax Efforts
May 01, 2024 | Wednesday | Features
Cervical cancer is the fourth most common cancer worldwide, and the second most common cancer among females in India. It is associated with the prevalence of human papillomavirus and lower socioeconomic status. According to the factsheet published by the ICO/IARC Information Centre on HPV and Cancer in 2023, India has a population of 511.4 million women aged 15 years and older who are at risk of developing cervical cancer. About 5 per cent of women in the general population are estimated to harbour cervical HPV-16/18 infection at a given time, and 83.2 per cent of invasive cervical cancers are attributed to HPVs 16 or 18.
The published report also highlights that in India, there are over 123,000 new cases and close to 77,000 deaths from cervical cancer annually. This translates to one woman dying of cervical cancer every eight minutes in India, even though cervical cancer is an almost completely preventable cancer. Let's explore how the country is poised to tackle HPV head on.
Image credit- shutterstock
As characterised and defined by the World Health Organisation (WHO), Human papillomavirus (HPV) is a common sexually transmitted infection that can affect the skin, genital area, and throat. Almost all cervical cancer cases (99 per cent) are linked to infection with high-risk HPV, an extremely common virus transmitted through sexual contact. Although most infections with HPV resolve spontaneously and cause no symptoms, persistent infection can cause cervical cancer in women.
Furthermore, experts worldwide gauge that typically, it takes 15–20 years for abnormal cells to become cancer, but in women with weakened immune systems, such as untreated HIV, this process can be faster and take 5–10 years. Risk factors for cancer progression include the grade of oncogenicity of the HPV type, immune status, the presence of other sexually transmitted infections, number of births, young age at first pregnancy, hormonal contraceptive use, and smoking.
According to WHO guidelines for the initiative of elimination of cervical cancer, all countries should reach and maintain an incidence rate of below 4 per 100,000 women. Achieving that goal rests on three key pillars and their corresponding targets: vaccination: 90 per cent of girls fully vaccinated with the HPV vaccine by the age of 15; screening: 70 per cent of women screened using a high-performance test by the age of 35, and again by the age of 45 and treatment: 90 per cent of women with pre-cancer treated and 90 per cent of women with invasive cancer managed. Only 1 per cent of women are screened for cervical cancer in India, as per a recent report. The rate of HPV vaccination in the country is suboptimal at present, due to several factors.
With the Indian government announcing the inclusion of the HPV vaccine in the national immunisation programme and the breakthrough development by Serum Institute of India (SII) in manufacturing India’s own HPV vaccine ‘Cervavac’, India can boost its efforts in achieving this 90-70-90 target and thereby blaze a brighter path for cervical cancer elimination.
As a priority, HPV vaccines should be given to all girls aged 9–14 years, before they become sexually active. The vaccine may be given as 1 or 2 doses. People with reduced immune systems should ideally receive 2 or 3 doses. Some countries have also chosen to vaccinate boys to further reduce the prevalence of HPV in the community and to prevent cancers in men caused by HPV.
Reducing healthcare burden
Vaccination is considered a cost-effective preventive measure compared to treating established cancers, saving healthcare systems significant resources.
The costs of cervical cancer treatment and management can vary significantly, influenced by factors such as the stage of cancer, geographical location, and healthcare infrastructure. Experts say that the treatment options for cervical cancer in India primarily include surgery, radiotherapy, chemotherapy, and palliative care, in addition to newly added technologically advanced treatment options. Screening leads to a reduction in the occurrence of cervical cancer cases with a decrease in cancer deaths, and the Visual Inspection with Acetic acid (VIA) every 5 years is the most cost-effective screening method in the context of India. Preventive intervention by HPV vaccination, especially at the right age, can contribute to reducing disease severity.
A 2020 study published in Lancet Oncology evaluating the potential survival effect of scaling up treatment showed that the cumulative effect of scaling up all imaging modalities together with expanded treatment and quality of care could improve 5-year net survival for cervical cancer to 62·5 per cent (globally). Investments in imaging equipment, personnel, and quality of care will be needed to scale up cervical cancer treatment successfully.
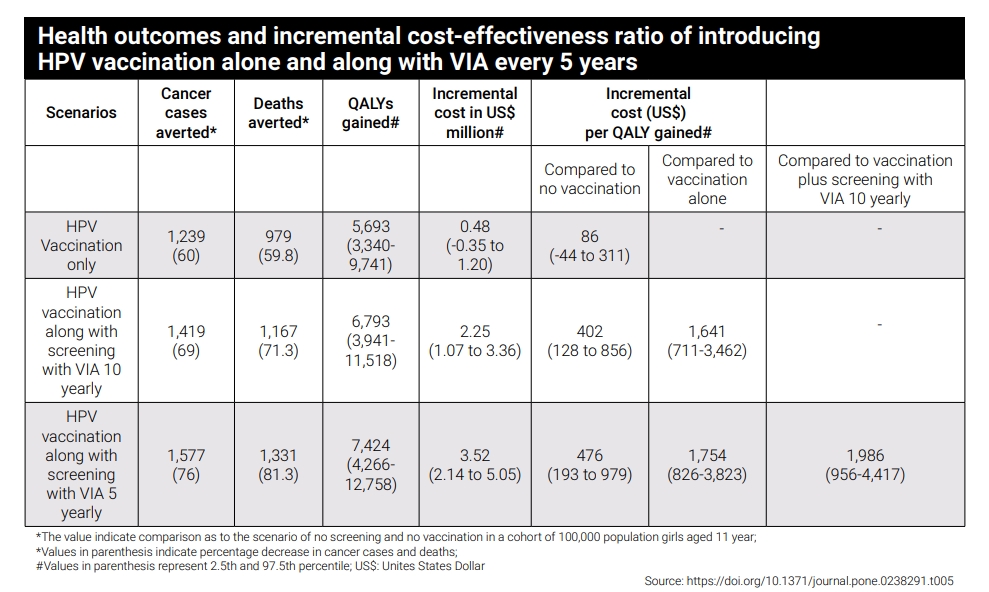
Additionally, according to a 2020 PLOS One study assessing the cost-effectiveness of various screening strategies for cervical cancer and HPV vaccination in India, immunising adolescent girls for HPV along with screening women using VIA appears to be a cost-effective strategy at both 5 yearly and 10 yearly frequencies of VIA screening, as compared to no vaccination and no screening. This indicates that successful vaccination may hold grand potential in the economical management of disease burden and this factor will be especially relevant in a country where cervical cancer burden is high. The inclusion of the cervical cancer vaccine in the national immunisation programme is a highly welcome move from the government.

Barriers and challenges
The most significant barriers being gauged by experts in achieving this large-scale vaccination will be the huge population to cover, lack of awareness combined with an overall vaccine hesitancy due to myths regarding vaccine safety, social/religious stigma, and vaccination costs.
Commenting on the cost factor for the vaccine candidates, Dr Smita Joshi, preventive oncologist and programme director at Jehangir Clinical Development Center, Pune, mentions, “Quadrivalent and nine-valent vaccine Gardasil by Merck and Cervavac by SII are the 3 vaccines available in India currently. Cervarix (bivalent HPV vaccine by GSK) has not been available in the Indian market for one year. SII’s HPV vaccine is slightly cheaper than Gardasil for the private market. It is supposed to be given to the government for Rs 300-500 per dose. However, it costs about Rs 1200 -1400 per dose in the private market.”
Furthermore, building the optimal infrastructure to execute the required scale and magnitude of HPV vaccination in the country and developing strategies for the sustenance of the vaccination programme are going to be additional key players in overcoming logistical hiccups. Reaching out-of-school girls to complete the vaccine regimens, poor tracking systems, and overall low rate of candidate adherence to vaccine regimen due to multiple doses (two to three doses may be recommended for Cervavac) can pose additional challenges in streamlining and monitoring the vaccination programme performance.
Dr Smita Joshi, preventive oncologist and programme director at Jehangir Clinical Development Center, Pune, stated, “Government will need proper implementation plan, powerful messaging, and communication strategy, ensure sustained funding and continuation of the programme, in addition to baseline political commitment. Monitoring screening and vaccination of out-of-school girls will be an important factor requiring adherence in ensuring maximal coverage for the vaccination drive.” She also added that with the current health infrastructure being overburdened, vertical investment is essential not only for vaccination but also for cervical cancer screening and management of precancers in adult women.
Estimating target populations and validating the availability of the necessary health infrastructure in the various parts of the country are important factors to consider when implementing a nationwide HPV vaccination initiative. Because of this, developing an evidence-based vaccination policy and guidelines for the new Cervavac HPV vaccine may prove crucial.
Reports indicate that in India, a significant proportion of cervical cancer cases come from rural areas. As compared to urban areas, access to facilities like regular screening, testing as well as general awareness regarding sexual health and sexually transmitted infections is considerably lower in India’s rural population. In the absence of these critical factors, covering the rural population optimally in implementing the nationwide vaccination programme might prove significant. In this regard, experts presume that the role of ASHA and Anganwadi workers in boosting HPV vaccination and fostering the right direction towards preventive healthcare (especially in rural areas) may be significant.
Additionally, given that developing evidence-based policies and guidelines will have benefits, consideration of the state-wise distribution of cervical cancer burden may become a significant factor for effective strategising. According to Dr Priya Kapoor, Consultant - Surgical Oncology (Gynaecology), Apollo Proton Cancer Centre Chennai, “More than 5 million women are at risk of developing cervical cancer. The age-adjusted incidence rate of HPV-related cancers is high in areas of Meghalaya and Assam. Delhi is also not far behind.”
Enabling equitable access to all socioeconomic groups to execute maximal vaccine coverage can drive India in the direction of truly reducing the disease burden resulting from HPV infections, which, although majorly includes cervical cancer caused by HPV subtypes 16 & 18, also comprises of conditions like anogenital warts, vaginal cancer, anal cancer, oropharyngeal cancer, etc.
All in all, collaborative efforts involving healthcare professionals, policymakers, and the public are crucial in raising awareness about the importance of HPV vaccination. Dispelling misconceptions, promoting accurate information, and ensuring widespread access to vaccination will lead to significant strides in preventing cervical cancer and enhancing women’s health in India.
Cervavac by SII
Although Serum Institute of India (SII)’s Cervavac was officially launched in 2022 and has been available in the market in private hospitals for nearly two years now, HPV vaccination has not gained the necessary momentum yet. High cost per dose and a probable limited community-wide reach of the private healthcare sector can be gauged as primary reasons. With the announcement of HPV vaccination to be rolled out for all girls aged 9-14 years and the government also planning to include the HPV vaccine in the national immunisation programme, the picture would change dramatically in the positive direction.
Remarking on the upcoming country-wide vaccination phase, Dr Umesh Shaligram, Executive Director of R&D, Serum Institute of India, said, “The government has a plan in place and a budget ready to begin the roll out for vaccination of girls aged between 9 and 14 years. The government might start the rollout in the third or fourth quarter of 2024. For that, Serum has got the back end covered and is poised to ensure efficient supply for this pilot phase of Cervavac mass vaccination.”
Elaborating on the timeline of upscaling Cervavac production to align with the government’s plans and goals, Dr Shaligram touched upon the role of COVID-19 on the supply chain logistics for large-scale HPV production, which was originally planned by SII to take off around 2019-2020. The pandemic not only disrupted the supply chain resulting in delayed deliveries of the required equipment from the West but it also raised constraints concerning space and resource allocation, as the need of the hour demanded a high priority to the manufacturing of COVID-19 vaccines. The post-pandemic recovery allowed us to reset the momentum and focus on the HPV vaccine, where SII focussed on building up the necessary infrastructure for Cervavac manufacturing from scratch. “It took almost a couple of years for us to get the infrastructure ready. Production set-up for scale-up of the Cervavac vaccine kickstarted in 2023, and now SII is all set to cater to the needs of the upcoming country-wide HPV vaccination”, added Dr Shaligram.
For the Indian market, available HPV vaccine candidates include the Merck vaccine Gardasil, the GSK vaccine Cervarix, and the indigenous Cervavac vaccine by Serum Institute of India. With some reports citing a potential discontinuation of the Cervarix vaccine from the Indian market, Gradasil and Cervavac would be the available choices in the long run.
Cervavac is very close in design and efficacy to the Merck vaccine, with both quadrivalent vaccines targeting the major HPV subtypes 16 & 18. Cervavac has also demonstrated protection against HPV 6 & 11 subtypes, which cause health conditions other than cervical cancer such as genital warts and cancerous lesions in other organs. In addition to the broad-spectrum efficacy of Cervavac, the advantages it incurs include the cost-effectiveness and the scale of vaccine availability for extended distribution owing to the strategic efficiency and manufacturing prowess of the Serum Institute of India.
According to the data provided by the Central Drugs Standard Control Organisation(CDSCO), the safety profile of Cervavac is based on data from clinical trial (SII-qHPV/IN-02) conducted in India where Cervavac was administered to 1530 study participants aged 9 through 26 years. The most common events occurring after Cervavac administration were injection site pain, and headache. The majority of adverse events were mild to moderate in severity and usually resolved within a few days of vaccination. Adverse events are organised by MedDRA System Organ Class (SOC).

Commenting on the rollout of Cervavac doses, Dr Shaligram added, “Considering the Indian birth cohort, which is up to 25 to 26 million per year, the number of doses recommended per individual and the estimated size of the target population of girls aged between 9 & 14 years, Serum Institute is looking at catching up to approximately 300 million vaccinations overall.” In addition to the cohort of girls aged between 9 & 14 years, the Cervavac vaccine can also be recommended for adolescent boys, as well as older men and women beyond 14 years of age up to 26 years of age. He also added that as vaccine advocacy and awareness improve, the government will shift focus to the older age groups and aim to cover that large cohort as well in the coming few years.
In view of the incorporation of the vaccine's rollout schedule and the government’s plans for implementing the vaccination programme, Dr Shaligram said that although a high demand for Cervavac is certainly expected, the government would look to implement the vaccination programme in phases and open up the country to HPV vaccination in parts. He added that Serum Institute is all set up to meet the increasing demands for Cervavac in India, and after covering the needs of India, Serum Institute will then look to go global with the Cervavac vaccine after some years.
Managing cervical cancer burden
The objectives of the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS), being implemented by the Central Government under the National Health Mission (NHM) for interventions up to the district level, include awareness generation for cancer prevention, screening, early detection and referral to an appropriate level institution for treatment, including cervical cancer. Screening of common NCDs including three common cancers i.e. oral, breast, and cervical is also an integral part of service delivery under Ayushman Bharat - Health and Wellness Centres.
Experts add that the path ahead to managing the cervical cancer burden also requires sustained collaboration between the government, healthcare organisations, NGOs, and communities to achieve comprehensive cervical cancer prevention.
Dr Smita Joshi, preventive oncologist leading the cervical cancer management operations at the NGO ‘Prayas’ in Pune shared, “Prayas has screened over 35,000 women and treated screen-positive women according to the WHO guidelines till date. However, our programme depends upon the donations that we receive. There are very few NGOs working in the field and everyone is doing their own small thing however a coordinated effort will help. However, for programme sustenance, better government policies are necessary.”
Under the NCD control plan, the PHCs are supposed to offer cervical cancer screening using VIA (whereas the WHO has suggested an HPV test). Treatment of cancers is also available under the Ayushman Bharat - Pradhan Mantri Jan Arogya Yojana (PMJAY). Under the umbrella scheme of Rashtriya Arogya Nidhi, financial assistance is provided to families living below the threshold poverty line for their treatment, including treatment of cancer, in government hospitals.
The ICMR-National Institute of Cancer Prevention and Research (NICPR) has made important strides in cervical cancer prevention. NICPR-ECHO training courses are continuing efficiently to train in-service healthcare providers in cancer screening and for capacity building of pathologists, especially in remote areas. The government is also collaborating with national and international healthcare organisations like WHO and UNICEF for technical assistance in planning, implementing, and monitoring the HPV vaccination programme.
However, it's good to know that a study published in 2022 in ‘BMC Cancer sheds light on the trends in the incidence and mortality of cervical cancer in India and its states between 1990 and 2019. The study is based on the data collected from various sources like the Global Burden of Disease study and the Bombay Cancer Registry and highlights that the incidence and mortality of cervical cancer declined over the past three decades, but also talks about how it is still a major public health problem in India.
Shivani Thakar
(shivani.thakar@biospectrumindia.com)












