Filter and Sensor Sets for Single-Use Filtration in Pharmaceutical Industry
November 05, 2020 | Thursday | Features
Innovative solutions from Sartorius enable the manufacturers to implement single-use filtration operation with ease, bypassing many in house requirements of validation, sterilization and assembly operations.
Amit Khanna, Manager for Separation Technologies (Asia)
Amit.khanna@sartorius.com
Amit comes from a Biotechnology background and has over 13 years of experience working in the field of Separation Technologies. He handles a team of Separation Technology Experts, gives product & application trainings to internal & external customers and has publications in leading Indian Industry journals. His expertise includes filter selection, scale up trials, integrity testing, process optimization & troubleshooting filter failures.
Traditional filtration operations in pharmaceutical industry involved Stainless steel-based lines, housings and associated sensors and other hardware. A typical operation would involve water flush, steam in place and post use clean in place procedures along with pre and post batch integrity testing. This further involves validation of steaming and cleaning procedures for filters and their re-use in the process.
In the last decade, manufacturers have moved towards single use of critical sterile filters and associated tubing set up in view of ever-increasing regulatory compliance on cleaning validation to prevent endotoxin or bioburden carryover. It also decreased the change over time for the campaign since no exhaustive cleaning and sterilization is required.
A single-use filtration set would require sterilization & assembly of filter, tubing and end connections along with the pressure resistant cable ties. All of these components these must be maintained in stock from separate vendors and then assembled correctly for sterilization and operation. Sterilization validation of a complex assembly may be difficult.

Image 1- Filter Transfer Set
Sartorius provides pre-assembled and pre-gamma-sterilized filter assemblies in double packing which is ready-to-use. This comes with 100% filter element testing, 100% visual inspection, biocompatibility according to USP 87 or 88 class VI, TSE | BSE free certification, endotoxin limits, sub visible particle limit, extractable profile documents, pre-qualified connections and ASTM transport validation. Sartorius uses Oetiker metal clamps wrapped in rubber which are more pressure resistant as compared to cable ties for securing connections. There is increased assurance of supply since a single vendor supplies all the parts in an assembly. With this, ordering and maintaining stock becomes easier. The pain of internal sterilization | cleaning validation along with the operating training for complex assembly is reduced. Assembly of sterile components traditionally requires LAF zone, which can now be eliminated. Customers can choose to configure these filter sets from a large selection of filter capsules, tubing make, tubing lengths and aseptic | sanitary connections.
Another important gap (when industry shifted form stainless steel-based filter lines) was the absence of point of use pressure and flow sensors for silicone tubing. A non-product contact arrangement was required between the reusable sensor hardware and the product solution. Sartorius offers innovative solutions for sensing POU pressure and flow.
A clamp on flow device which uses two pairs of ultrasound source and sensors which measure the transit time difference between two signals (upstream and downstream sensor) to determine flow velocity. No part of the sensor is in actual contact with the product. A single-use flow pipe is provided which increases the accuracy over using bendable silicone tube. The sensor comes pre-calibrated and has high accuracy for the chosen flow range and pipe ID. The signal can be displayed on the amplifier box and further connections can be made into SCADA system for capture of process data.
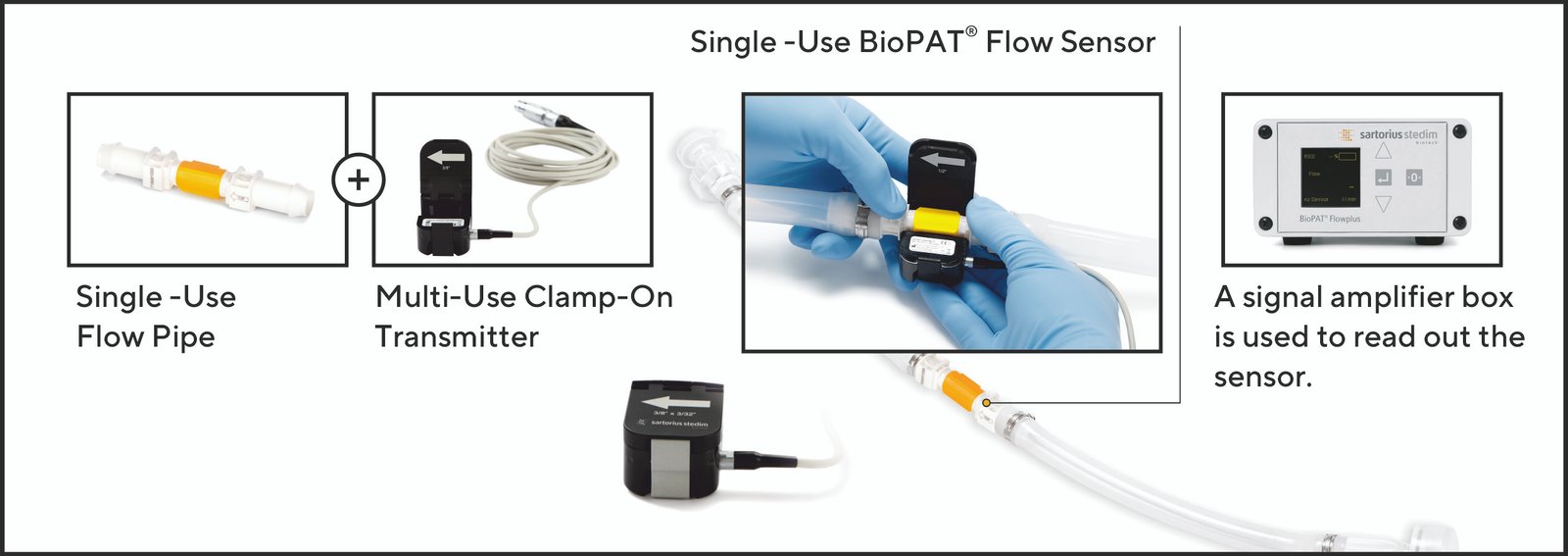
Image 2: BioPAT® Flow sensor is a combination of single-use flow pipe and a multi-use clamp-on
Pressure measurement in a single use arrangement is provided by combining multi use pressure transmitter with single-use pressure pipe. The silicon membrane of the pipe and the metal membrane of the transmitter get tightly connected through an integrated fixing mechanism. The pressure in the pipe bends the silicon membrane, which bends the metal membrane and the exerted force is detected by a pressure sensor within the transmitter. An independent display for pressure or input to SCADA system can be configured at the user site.
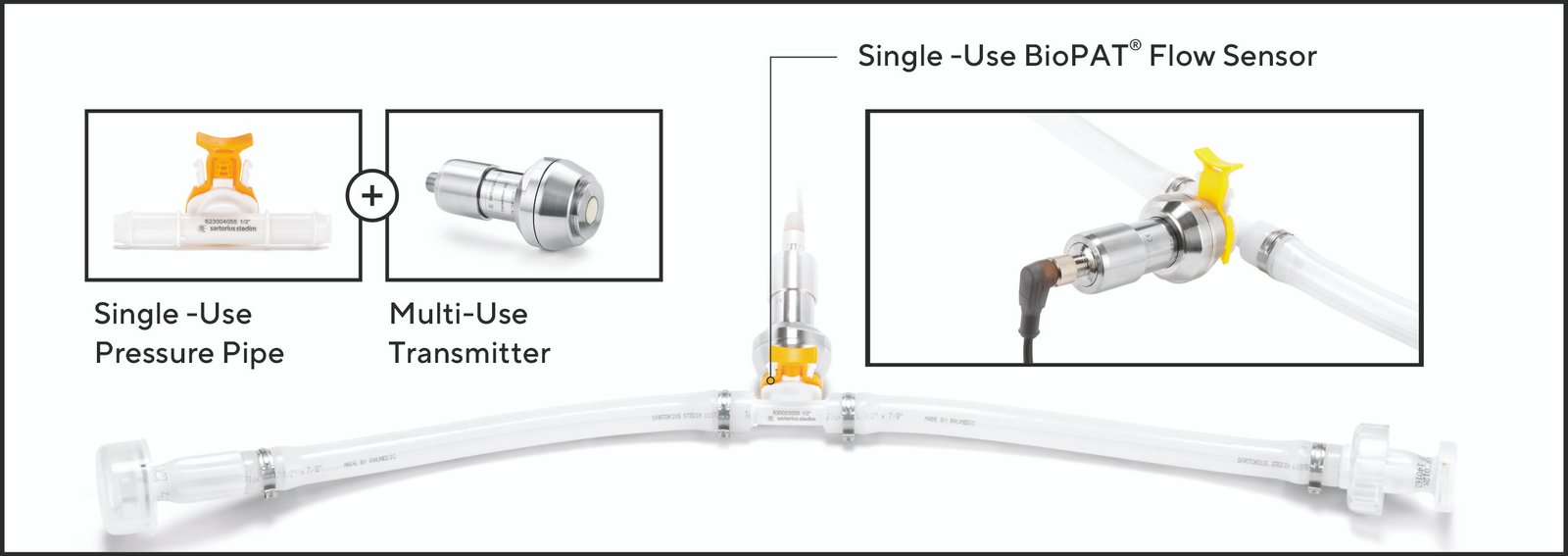
Image 3: BioPAT® Pressure sensor is a combination of single-use pressure pipe and multi-use transmitter
There are specific needs that require special single-use assemblies for e.g PUPSIT arrangement. Sartorius enables the development of customized solutions with the support of a team of application specialists who study the process requirments and conceptualize the prototype design. The prototype is further tested and modified, if required, for final approval and supply.
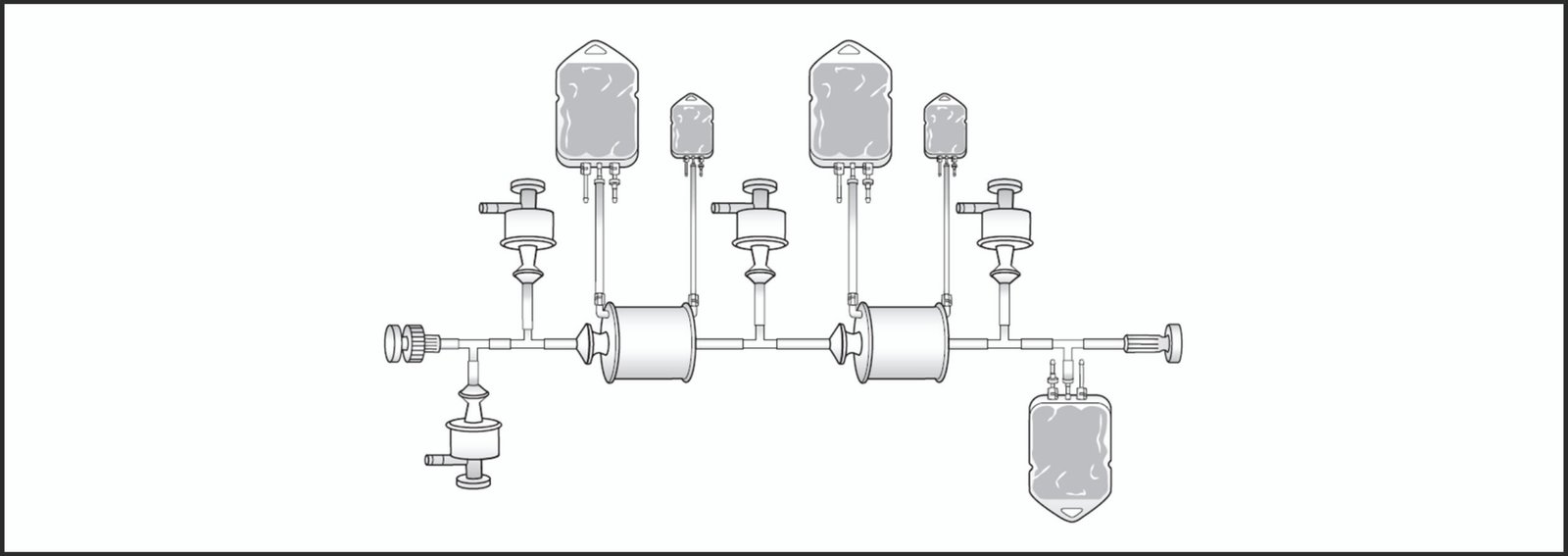
Image 4: Development of customized solutions is possible
Innovative solutions from Sartorius enable the manufacturers to implement single-use filtration operation with ease, bypassing many in house requirements of validation, sterilization and assembly operations. Single-use sensor sets with pre-calibrated hardware further provides the required operating parameter monitoring and data capture at point of use without compromising the aseptic nature of the process.
For further information, visit:
www.sartorius.com













