TakeOne®- the only aseptic sampling system of it’s kind
October 06, 2020 | Tuesday | Features
TakeOne® Aseptic sampling device is completely ready to use & have multiple, independent sampling lines
Not all samples are equal – categorization of a sampling plan by assay type reduces costs, improves sampling results and optimizes capacity of the sampling solution. Samples are drawn throughout the entire Pharma | Biopharmaceutical process for different steps like compounding, holding stage, cell expansion, fermentation, buffer | media preparation, purification, final fill finish etc.
Fetaures and benefits:
- Single use – eliminates cross contamination.
- Pre-assembled & ready to use – saving time and increasing safety.
- 100% leak tested and Gamma sterilized.
- Available tank fittings – 25mm InGold , 1.5” and 2” TC
- TakeOne® Flex – sampling from single use containers.
- Quick and easy disconnect.
- No sample overflow- spring return actuator.
- Application driven containers – Centrifuge tubes, PETG bottles, EVA & PE bags , needle free sampling etc.
- Manifold availability – expand sampling capacity.

Image 1: TakeOne® Aseptic Sampling System ensures that sample and product integrity is preserved
TakeOne® -Aseptic sampling device has been engineered to take perfectly representative samples giving exact results with no risk of false-positives for each process step, thanks to its pre-assembled, sterile and closed design. It collects process fluids for analysis while protecting the process fluid from adventitious agents.
Microbiological assays like bioburden and endotoxin monitoring is required to demonstarte process control to prevent a batch rejection due to loss of sterility or high bioburden and compliance of the final drug product to the limits set by regulatory agencies; the process monitoring assays ensure that critical parameters like viability, osmolality, immunoglobulin, nutrients, conductivity, pH and gas analysis are met for successful drug production.
TakeOne® Aseptic sampling device is completely ready to use & have multiple, independent sampling lines which allow for collection of perfectly representative samples. Perfectly representative samples provide perfectly reliable data allowing operators to quickly react to out-of-specification results.
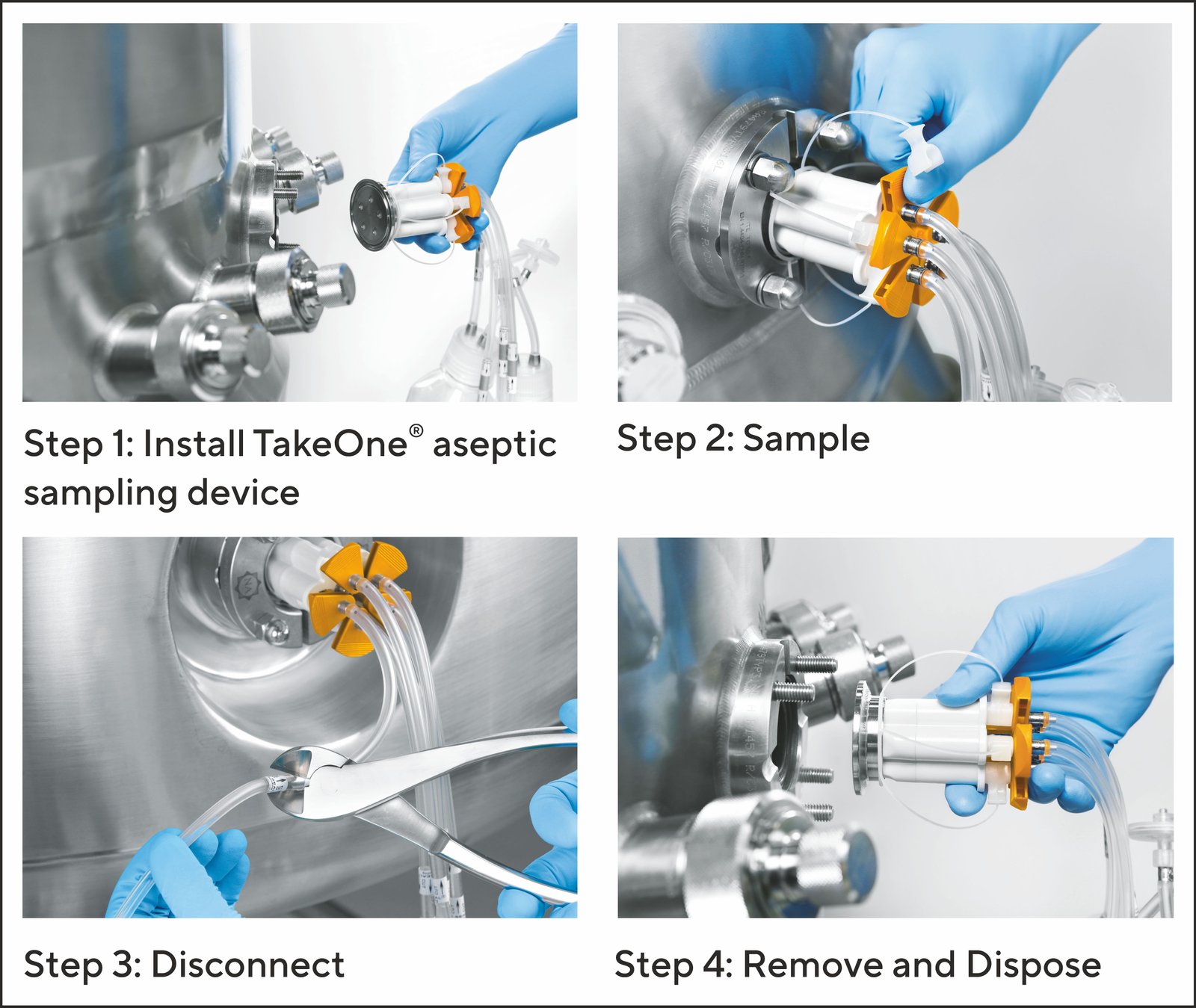
Image 2: Efficeint sampling with TakeOne® Aseptic Sampling System
During actuation the cannula, or the needle pierces the self sealing septa that is molded to the faceplate allowing fluid to enter the sampling pathway and into the collection vessel. The design and operation of cannula diaphragm and septa ensure a closed pathway before, during and after actuation.
Once the sample has been collected operators easily cut the aluminum collar with a hand held cutting tool aseptically disconnecting the tubing and the sampling container.

Image 3: TakeOne® Aseptic Sampling System allows for point of use configuration
For further information, visit:
www.sartorius.com
Authors

Niveditha Shetty
Application specialist | Fluid Management Technologies
Niveditha.Shetty@Sartorius.com
Niveditha has a master’s degree in biotechnology from Bangalore University and close to a decade of experience in biopharmaceutical industry focusing on the single use applications. Niveditha has had a successful tenure at Pall Corporation and Saint Gobain before her current assignment at Sartorius.

Kiran Bhatambarekar
Application specialist | Fluid Management Technologies
Kiran.Bhatambarekar@Sartorius.com
Kiran is a microbiologist by training and has ten years of experience working in the Biopharmaceutical industry He is now involved in establishing Sartorius’ Fluid Management product portfolio in the Indian Pharma & Biopharmaceutical market. Kiran’s expertise lies in end to end single-use solutions, upstream solutions & troubleshooting.














