Enhanced Assurance of Supply (for Single-Use Bags)
October 01, 2019 | Tuesday | Features | By Apala Banerjee Product Specialist- Fluid Management Technologies
Sustainable supply chain helping you ensure patient safety & long term supply
Apala Banerjee Product Specialist- Fluid Management Technologies
Apala is a microbiologist by training and has been involved in establishing Sartorius Stedim Biotech’s Fluid Management Technologies portfolio in the Indian biopharmaceutical market. Apala has a background in vaccine manufacturing.
Sartorius Stedim Biotech introduced the first single-use bag for biopharmaceutical applications and now we see rapid adoption of single-use bags in commercial production of biopharmaceutical drugs. The entire supply chain for single-use bags is complex and requires material science and film expertise to achieve consistent quality attributes of film, bags and single-use assemblies. This raises new challenges for bag suppliers and drives the need for consistent product quality, improved assurance of supply, robust change management and business continuity planning. Sartorius Quality by Design, material science, film extrusion knowhow and bag making expertise are the foundation of our single-use solutions that offer robustness, performance, process safety and cost effectiveness. Assurance of Supply (AOS) for our SU bags are built on partnerships, supply contracts, quality agreements and the control of our entire manufacturing processes from the resins to the final product (refer image 1).
Resin specifications and process controls for our PE film of Flexsafe® and EVA film of Flexboy® and Celsius® provide
- Raw material traceability
- Lot-to-lot consistency
Extrusion design space and process control for films provide
- Excellent & reproducible cell growth
- Consistent robustness
- Lot-to-lot reproducible extractable profiles
Long-term supply contracts, safety stocks & quality agreements provide
- 10-year supply contract for films and 2-year customer change notification
- 2-year last time buy option for films
- 2 years of demand safety stocks for resins & films
- 2 years change notification for fluid contact components
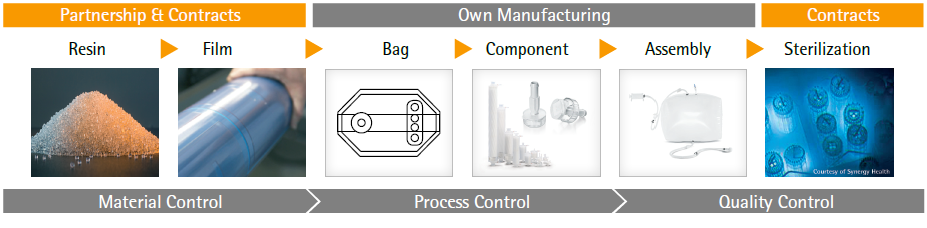
Supply chain for single-use commercial manufacturing needs to be robust as any change may affect the quality of the drug product. This is why Sartorius Stedim Biotech has an extremely stringent change control in order to offer our customers Assurance of Supply (AOS). This industry leading Assurance of Supply (AOS) is the result of a global supply network and expansion of our manufacturing footprint worldwide. We have built strong partnerships and established long-term quality & supply agreement with key suppliers. By controlling our entire supply chain and manufacturing process, we ensure consistent quality, change control and busines










